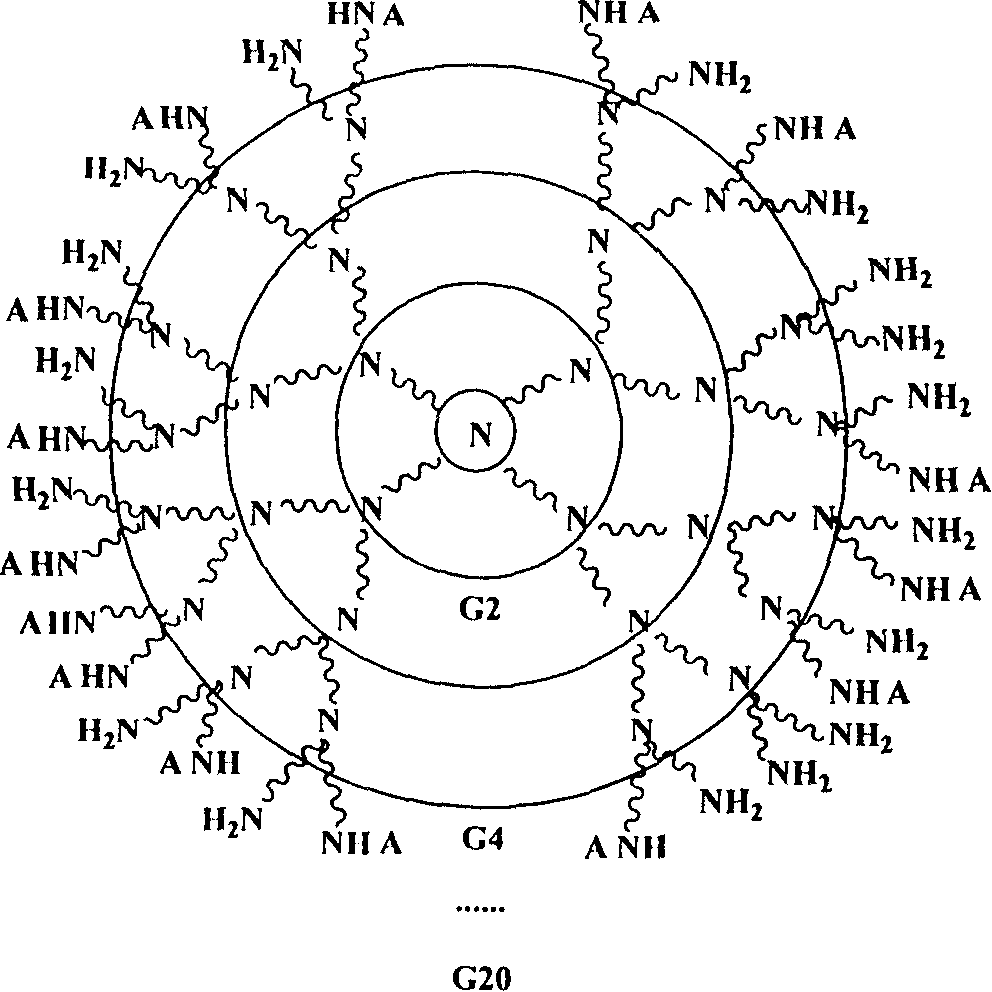
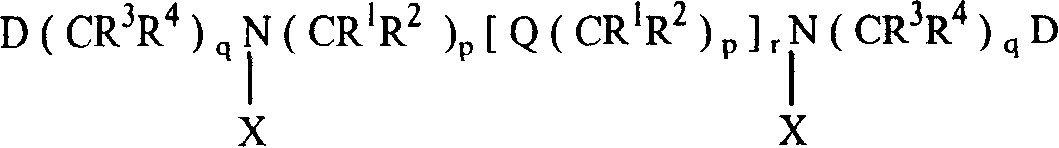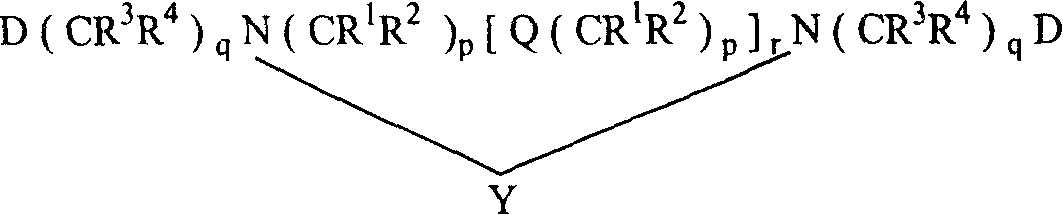Tree shape Macro molecule paramagnetic metal complex and synthetic method and use
A paramagnetic metal and macromolecular technology, applied in the field of dendritic macromolecular paramagnetic metal complexes and their synthesis and use, can solve the problems of short retention time of Magnevist, no tissue or organ selectivity or targeting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Dissolve 0.5g dendrimer G1 and 5mL triethylamine in 60mL distilled water, cool with ice salt at -20°C, slowly add 1.68g bromoethane-substituted 1,4,7,10-azacylidene 30ml dimethylformamide DMF solution of alkanetetraacetic acid DOTA, react at low temperature for 4h, rise to room temperature and continue to react for 48h, filter, evaporate part of the solvent under reduced pressure, cool to room temperature, pour the residual solution into 600mL absolute ethanol- In the mixed solvent of anhydrous ether, the volume ratio of absolute ethanol: anhydrous ether is 1:2, a light yellow precipitate is produced, filtered, and drained, and the obtained solid is dissolved in distilled water again, with absolute ethanol or anhydrous Diethyl ether was reprecipitated, and the pale yellow solid was collected and dried in vacuo to obtain 1.84 g of G1-DOTA-PM with a yield of 84.6%.
[0064] 1.84g G1-DOTA was dissolved in 20mL double distilled water, under stirring, add 0.59g gadolinium ch...
Embodiment 2
[0066] Dissolve 1.4g of dendrimer G2 and 5mL of triethylamine in 60mL of distilled water, cool with ice salt at -10°C, slowly add 1.82g of bromoethane DOTA-HP in 30ml of DMF solution dropwise under stirring, react at low temperature for 4h, at room temperature Continue to react for 48 hours, filter, evaporate part of the solvent under reduced pressure, cool to room temperature, pour the residual liquid into a mixed solvent of absolute ethanol-anhydrous ether under stirring, the volume ratio of absolute ethanol: absolute ether is 1:2 , resulting in a light yellow precipitate, which was filtered and drained, and the obtained solid was reprecipitated with distilled water-ethanol / ether, and the light yellow solid was collected and vacuum-dried to obtain 2.74g of G2-DOTA-HP with a yield of 85%.
[0067] 2.74g G2-DOTA-HP was dissolved in 20mL double distilled water, under stirring, add 0.25g ferric chloride FeCl 2 ,, the reaction solution was reacted at room temperature for 1 h, adj...
Embodiment 3
[0069]1.2g dendrimer G5 was dissolved in an appropriate amount of water, cooled with ice water, and added in batches with 8.78g p-thiocyanate benzyl-DOTA 30ml DMF solution under stirring, reacted at low temperature for 4h, continued to react at room temperature for 48h, filtered, and the filtrate was reduced Part of the solvent was removed by pressure evaporation, cooled to room temperature, and the residual liquid was poured into a mixed solvent of absolute ethanol-anhydrous ether under stirring. , pumped to dryness, and the resulting solid was reprecipitated with distilled water-ethanol / ether, and the light yellow solid was collected and vacuum-dried to obtain 7.48g of G5-DOTA, with a yield of 75%.
[0070] 7.48g G8-DOTA was dissolved in 20mL double distilled water, under stirring, add 0.25g manganese chloride MnCl 2 , the reaction solution was reacted at room temperature for 1 h, adjusted to pH 5 with dilute sodium hydroxide solution, continued to stir for 12 h, filtered, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com