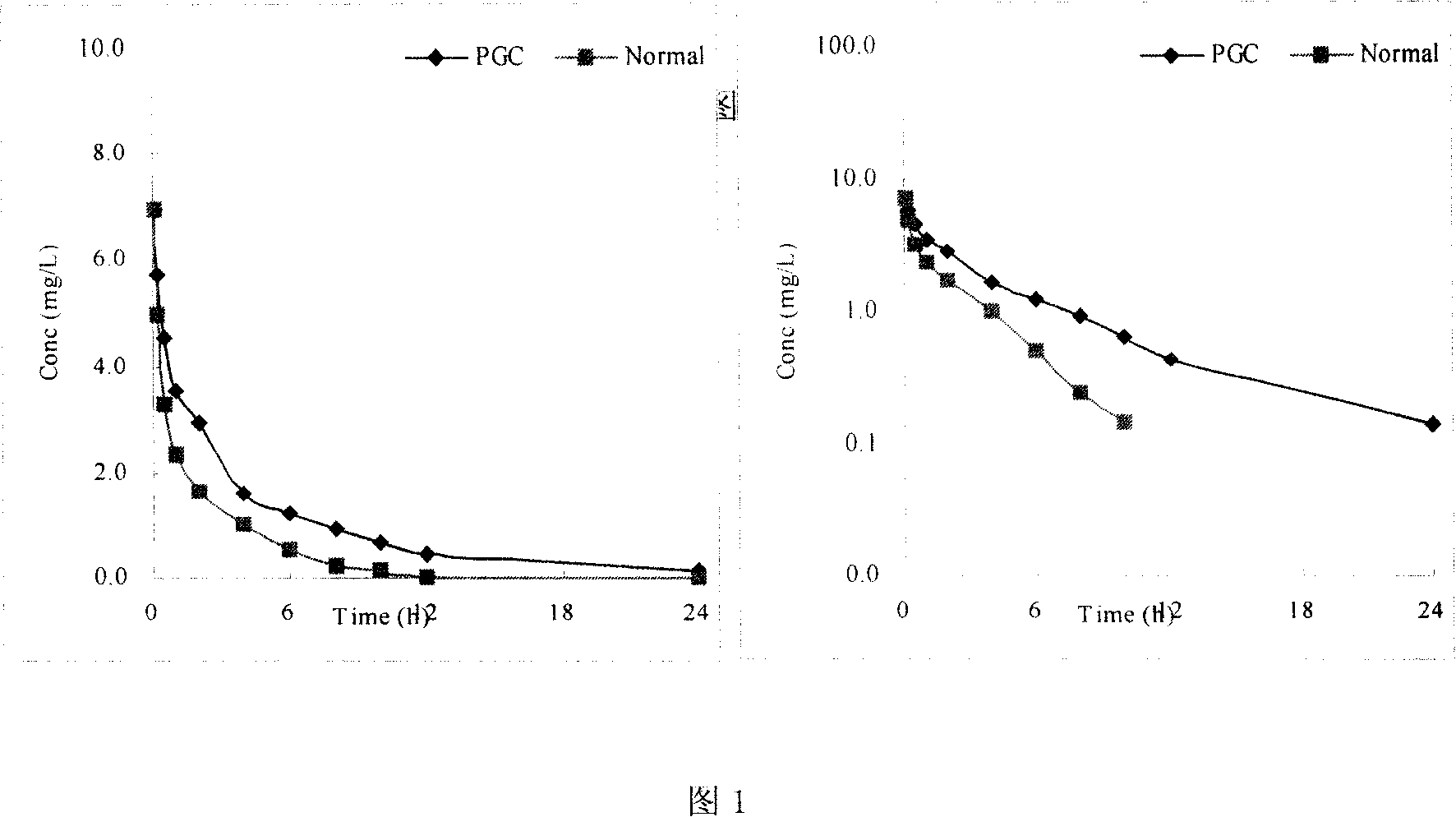
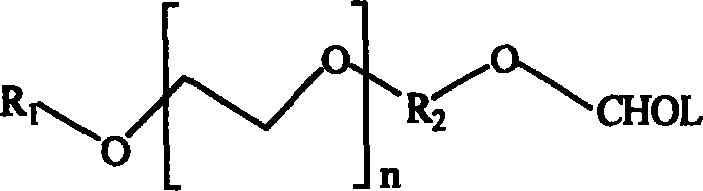
Polyethylene glycol modified sterol copolymer and its uses
A polyethylene glycol and copolymer technology, applied in the field of medicine, can solve the problems that it cannot be used as an auxiliary material for injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment one (subnanometer emulsion type)
[0089] Prescription 1: paclitaxel 0.01%-3.0%, co-solvent 0.01%-5.0%, phospholipid 0.5%-6.0%, PGC 0.1%-5.0%, triglyceride 5%-30%, glycerin 1.0%-6.0%, Oleic acid 1.0% ~ 6.0%, add water for injection to 100ml.
[0090] Weigh 100-500 mg of paclitaxel, dissolve it in an appropriate amount of co-solvent (absolute ethanol) 1, dissolve it in 15 g of triglyceride and 0.1-5 g of oleic acid, stir at 50 ° C to 80 ° C at high speed to mix evenly, and make an oil phase; Remove ethanol by evaporation; weigh 1.0 g of egg yolk lecithin, 1.0 g of PGC, and 3 g of glycerin, add the prescribed amount of water, stir at 50°C to 80°C at high speed to fully disperse, and make an aqueous phase. Mix the two phases of oil and water, and stir at a high speed at 50°C to 80°C to make colostrum. Take the colostrum, adjust the volume to the prescribed amount with water for injection, adjust the pH value to 5.0-7.0, transfer to the high-pressure homogeni...
Embodiment 2
[0091] Embodiment two (nanometer microemulsion formulation)
[0092] Prescription 1: Doxorubicin 0.01%-2.0%, Adriamycin co-solvent 0.01%-5.0%, PGC 0.1%-5.0%, co-surfactant (absolute ethanol, propylene glycol) appropriate amount, add water for injection to 100ml .
[0093] Weigh 100-500mg of doxorubicin and dissolve it in doxorubicin co-solvent (absolute ethanol), add PGC2.0g, 1.0g of propylene glycol and appropriate amount of water and stir at 20°C-80°C to mix evenly, keep stirring, add appropriate amount of no Titrate with water and ethanol until it becomes a translucent solution, adjust the pH value to 5.0-7.0, sterilize, filter, fill with nitrogen, and sterilize.
Embodiment 3
[0094] Embodiment three (micelle)
[0095] Prescription 1: Nimodipine 0.01%-2.0%, nimodipine co-solvent 0.01%-5.0%, PGC 0.1%-5.0%, polyoxyethylene-polyamino acid copolymer appropriate amount, and water for injection is added to 100ml.
[0096] Dissolve nimodipine, PGC, and polyoxyethylene-polyamino acid copolymer with an appropriate amount of solvent, evaporate the solvent under reduced pressure, add the prescribed amount of aqueous solution, stir evenly, and stir at high speed until the average particle size of the emulsion drops is ≤0.5 microns. After the emulsion is filtered, Nitrogen filled filling, sterilization vested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com