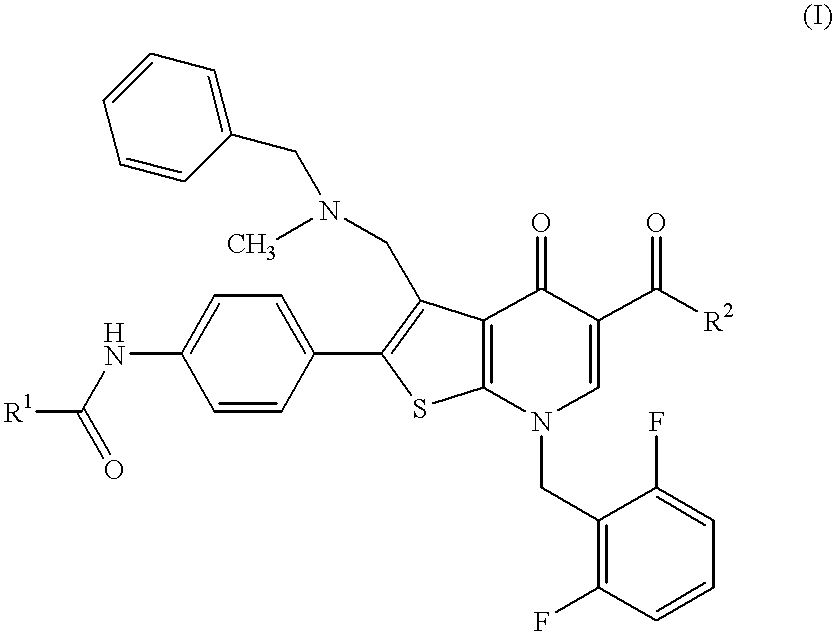
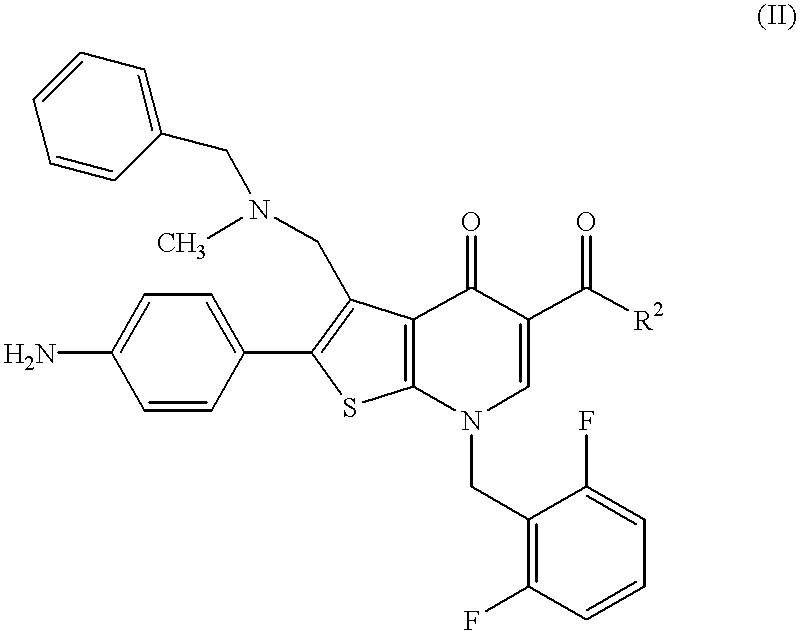
Thienopyridine compounds, their production and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
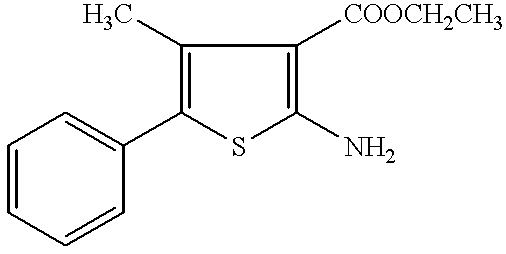
Production of 2-amino-5-phenylthiophen-3-carboxylic Acid Ethyl Ester
137. To a mixture of ethyl cyanoacetate (6.1 g, 50 mmol), sulfur (1.61 g, 50 mmol), triethylamine (3.5 ml, 25 mmol), and dimethylformamide (10 ml), phenylacetaldehyde (50% solution in diethyl phthalate; 12.05 g, 50 mmol) was added dropwise with stirring at 45.degree. C. over 20 minutes. After stirring at 45.degree. C. for 9 hours, the reaction mixture was concentrated and the obtained residue was extracted with ethyl acetate, washed with saline and dried (MgSO.sub.4), the solvent was distilled off under reduced pressure. The residue was chromatographed on silica gel and recrystallized from ether-hexane to yield pale yellow tabular crystals (5.55 g, 45%).
138. mp 124.5-125.5.degree. C. (lit.; 123-124.degree. C.).
139. Elemental analysis for C.sub.13H.sub.13NO.sub.2S C (%) H (%) N(%)
140. Calculated 63.13; 5.30; 5.66
141. Found: 62.99; 5.05; 5.63
142. .sup.1H-NMR (300 MHz, CDCl.sub.3) .delta.: 1.37 (3H, t, J=7.1 Hz), 4.30 ...
reference example 2
Production of 2-amino-4-methyl-5-(4-methoxyphenyl)thiophen-3-carboxylic Acid Ethyl Ester
144. A mixture of 4-methoxyphenylacetone (16.5 g, 0.10 mol), ethyl cyanoacetate (12.2 g, 0.10 mol), ammonium acetate (1.55 g, 20 mmol), acetic acid (4.6 ml, 80 mmol), and benzene (20 ml) was heated and refluxed for 24 hours, while the water being produced was removed using a Dean-Stark apparatus. After cooling, the reaction mixture was concentrated under reduced pressure and the residue was partitioned between dichloromethane and aqueous sodium bicarbonate. After the organic layer was washed with saline and dried (MgSO.sub.4), the solvent was distilled off under reduced pressure. To a solution in ethanol (30 ml) of the obtained residue, were added sulfur (3.21 g, 0.10 mol) and diethylamine (10.4 ml, 0.10 mol), followed by stirring at 50 to 60.degree. C. for 2 hours, successively the reaction mixture was concentrated. The obtained residue was extracted with ethyl acetate, washed with saline and dr...
reference example 3
152. Using some acetone derivatives in place of 4-methoxyphenylacetone, the following compound was obtained in the same manner as in Reference Example 2. 3
153. Yield: 40%
154. mp 64-65.degree. C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| reaction time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com