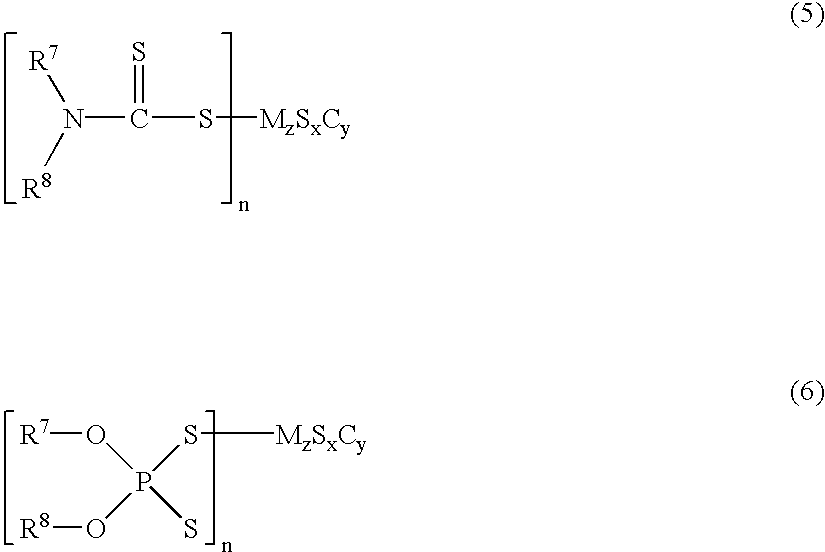
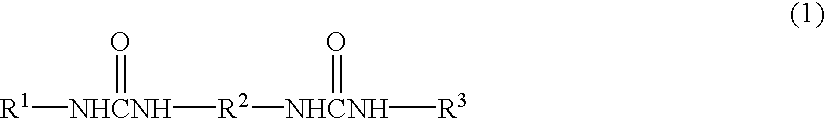It is generally known that incorporation of water into the lubricant results in great reduction in durability.
However, in case that the contact rubber seal undergoes deterioration or damage, water can seep into the chock and then into the lubricant of the bearing.
These electric part and accessory bearings are subject to invasion by muddy water or rain water splashed up from a road, and the water pump bearing is subject to invasion by circulating water for engine cooling.
That is, where vibrations are applied during running, the oily film formed between raceways and rolling elements becomes insufficient to impose a tensile stress to the contact area, and where a rotating shaft and the inner race are fitted with a strong
interference fit to reduce the rigidity of the bearing housing, a tensile stress always acts on the raceways.
As a result, the bearing may tend to undergo early flaking under the influences of the
water content originally present in the lubricant even with no water incorporated from the outside into the lubricant, resulting in a reduced bearing life L.
Therefore, these bearings are very susceptible to the influences of the vibrations and the like.
Bearings for automobile wheels are subject to invasion by water of muddy water or rain water on a road into their lubricant.
Bearings for guide rolls of
continuous casting equipment of iron and steel materials and those for back-up rolls of a
rolling mill are also subject to invasion by cooling water or rolling water into the lubricant.
Bearings for drier rolls of a
paper machine are subject to
attack of steam because they are used in a step of
drying water-containing wet paper and are therefore liable to early fracture on account of the increased
water content in the lubricant (see W. J. Culter, TAPPI Journal, Vol. 79, No. 2, pp.
However, since bearings for drier rolls of a
paper machine are usually used under a high temperature condition, it is difficult to apply a contact rubber seal as used in the bearings for the
work roll or the bearings for automotive wheels from considerations for
heat resistance.
A later survey of
actual use of
work roll bearings found that seizure accidents reduced drastically, but it turned out that the time until flaking, i.e., the bearing life L was not so improved.
It seems that the reduction of seizure accidents is attributed to reduction of leakage of the lubricant by the contact rubber seal fitted in the inside of the bearings and that the failure to improve the bearing life L is because water seeps in the lubricant to greatly reduce the rolling fatigue strength of the bearing.
That is, the first prior art fails to completely prevent water from seeping into the lubricant.
The rolling fatigue strength of the bearing material reduces as a result, failing to secure durability with a desired bearing life L.
The problem of the second prior art, however, lies in the difficulty in achieving prevention of water's seeping to such an almost perfect degree that the
water concentration in the lubricant be kept at 100 ppm or lower.
Accordingly, it meets difficulty in controlling the
water concentration in the lubricant at or below 100 ppm as stated above, thus failing to obtain desired durability.
It follows that the grease according to the fourth prior art softens to reduce its vibration
damping capacity, resulting in a failure to prevent early flaking of the bearing.
The early flaking combined with water incorporated into the lubricant can cause reduction in bearing life, failing to obtain desired durability.
Size reduction of ancillary engine parts is unavoidably accompanied with a reduction in output.
Further, the increased tension of the driving belt has been increasing the load on the bearings.
In cases where bearings for electric parts and accessaries are used under such high temperature and high speed conditions,
softening of grease is accelerated to reduce seizure resistance of the grease, and the
damping capacity of the grease also decreases to incur early flaking of the bearings, resulting in a failure to obtain desired durability.
The fifth prior art is in principle similar to the first prior art in respect of use of a contact rubber seal and therefore has the difficulty in perfectly preventing water's seeping.
Stainless steel has lower heat
conductivity than low
alloy steel and is therefore more liable to seizure fracture.
Therefore, it is difficult to apply stainless steel to those bearings which are used under the above-described poor lubricating conditions that may allow water to enter the lubricant.
Furthermore, since stainless steel is hardened at a high temperature from 1010 to 1070.degree. C. in the production of bearings, a
salt bath furnace should be used as a
heating furnace, which may incur an increase of cost of production facilities (see Nihon Tekko Kyokai (ed
Additionally the
grinding speed of stainless steel is lower on account of the lower heat
conductivity as described above, which increases the
grinding cost.
The stainless steel itself, being high
alloy steel, increases the material cost.
Although it is known, as previously described, that the state of the lubricant's containing water causes reduction of rolling fatigue strength of the bearing material, there is no established theory about this phenomenon.
Where water enters a lubricant, even a trace amount of water can make oily film formation difficult, and rolling elements and races undergo
metal-to-
metal contact between the rolling surface and the raceway surface.
The surfaces of the rolling elements and races are not homogeneous, and non-metallic inclusions such as oxides and sulfides are unavoidably formed on the rolling surface or raceway surface.
This cause
corrosion reaction to occur in that small crevice.
Corrosion of this kind is apt to develop particularly on the raceway surface of the outer race.
The water content originally present in the lubricant may induce
corrosion reaction even if outside water does not seep in.
The
corrosion reaction product clogs the inlets to the crevices so that
oxygen is hardly supplied from the surface to the crevices.
The bearing material thus undergoes
hydrogen embrittlement and reduces its rolling fatigue strength, which causes flaking and reduction of the bearing life L.
If the
hydrogen ion exponent pH exceeds 13 on the other hand, caustic corrosion develops to wear the raceway surface and rolling surface, and vibrations during running of the bearing gradually becomes noticeable.
However, where the inner race and the rotating shaft are an
interference fit, a tensile stress is always exerted on the raceway of the rotating race.
It is not only technically difficult but economically disadvantageous to control
corrosion reaction by using such high
alloy steel as stainless steel (SUS 440C) as a bearing material as previously mentioned.
It may be seen as unnecessary to define the upper limit of the content, but the compounds used as a reaction
film forming agent are relatively expensive, and excessive addition of the reaction
film forming agent may tend to accelerate reaction with the bearing material abnormally to induce corrosion or abnormal wear.
If the
hydrogen ion concentration of the lubricant is high, that is, if the
hydrogen ion exponent pH is low, the rate of production of corrosion products becomes high so that the reaction film is not sufficiently formed in the crevice, resulting in a failure to sufficiently suppress
hydrogen absorption inside the bearing material.
On the other hand, even where a
polyol ester oil, which exhibits the most excellent
lubrication characteristics of the synthetic base oils, is used, if water seeps into the lubricant and the hydrogen
ion exponent pH exceeds 13, there is a possibility that the
base oil undergoes deterioration by
hydrolysis.
If the average particle size is greater than 2 .mu.m, such particles as act as
foreign matter increase to accelerate wear of the rolling surface or the raceway surface, tending to cause early damage of the bearing or to deteriorate acoustic characteristics of the bearing.
As mentioned above, addition of particles comprising an
inorganic compound to the lubricant contributes to improvement in bearing durability, but the effect as expected cannot be obtained sufficiently if the amount added is less than 0.001 wt %.
If, on the other hand, the amount exceeds 3 wt %, the
inorganic compound particles increase in number to accelerate wear, which adversely affects the seizure resistance.
However, similarly to the second mode, mere addition of the particles is not enough.
If the hydrogen ion concentration of the lubricant is high, that is, if the hydrogen ion exponent pH is low, the rate of production of corrosion products becomes high so that a strong reaction film is not sufficiently formed in the crevice, resulting in a failure to sufficiently suppress
hydrogen absorption inside the bearing material.
If the aromatic ring
molar ratio Z is less than 0.5, the lubricant is liable to leak outside.
That is, leakproofness of the lubricant is not secured.
If the thickener content in the lubricant is less than 8 wt %, the gelling ability is insufficient for obtaining sufficient
hardness, and lubricant leakage increases.
If the content exceeds 35 wt %, on the other hand, the durability under high temperature and high speed conditions is deteriorated noticeably.
 Login to View More
Login to View More