Method of imaging lymphatic system using nanocapsule compositions
a composition and nanocapsule technology, applied in the field of nanocapsule compositions for imaging the lymphatic system, can solve the problems of nerve damage risk, reduced immune system function, and selective lymph node dissection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Albumin Coated Polycaprolactone Nanobubbles
[0053] A 6% aqueous solution was prepared from a 25% solution of USP grade human serum albumin (HSA) by dilution with deionized water. Separately, 1 part by weight polycaprolactone and 5 parts cyclooctane were dissolved in 55 parts isopropyl acetate at approximately 70° C. Once dissolution was complete, the organic solution was then thoroughly emulsified into an equal volume of the prepared HSA solution using a rotor / stator homogenizer. The emulsion was then diluted into 17 volumes of deionized water maintained at 30° C. and containing glutaraldehyde to crosslink the HSA. During the addition, the pH of the bath was monitored to insure that it remained between 7 and 8. Low shear mixing was continued for approximately 2½ hours until the isopropyl acetate had completely volatilized. Poloxamer 188 was then dissolved into the bath. Final concentration of the poloxamer was 0.25%. The suspension was allowed to come to room temperat...
example 2
Preparation of Albumin Coated Polylactide Nanobubbles
[0056] A 50.0 gm 6% aqueous solution was prepared from a 25% solution of USP grade human serum albumin by dilution with deionized water. Separately, a 25 gm organic solution containing 0.98% poly-d,l-lactide, 6.91% cyclooctane, and 92.1% isopropyl acetate. The organic solution was then thoroughly emulsified into the prepared aqueous solution using a Virsonic sonicator homogenizer. The emulsion was then diluted into 350 ml deionized water maintained at 30° C. and containing 1.25 ml of 1N NaOH. After the emulsion was fully dispersed, 1.0 ml 25% gluteraldehyde was added to crosslink the HSA. During the addition, the pH of the bath was monitored to insure that it remained between 7 and 8. Low shear mixing was continued for approximately 2½ hours until the isopropyl acetate had completely volatilized. Poloxamer 188 in the amount of 0.75 gm was then dissolved into the bath. The suspension was allowed to come to room temperature and the...
example 3
In-Vitro Acoustic Study
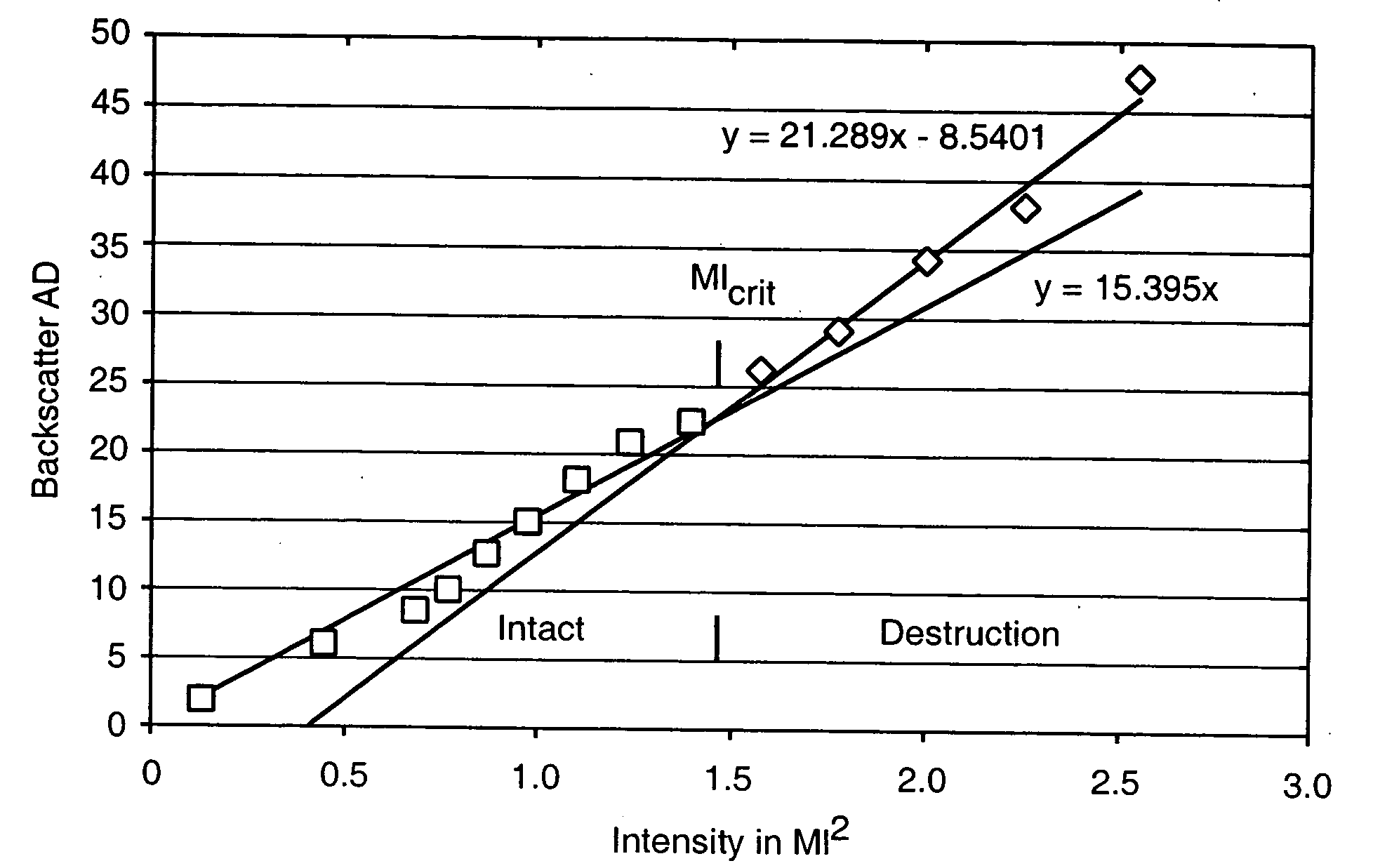
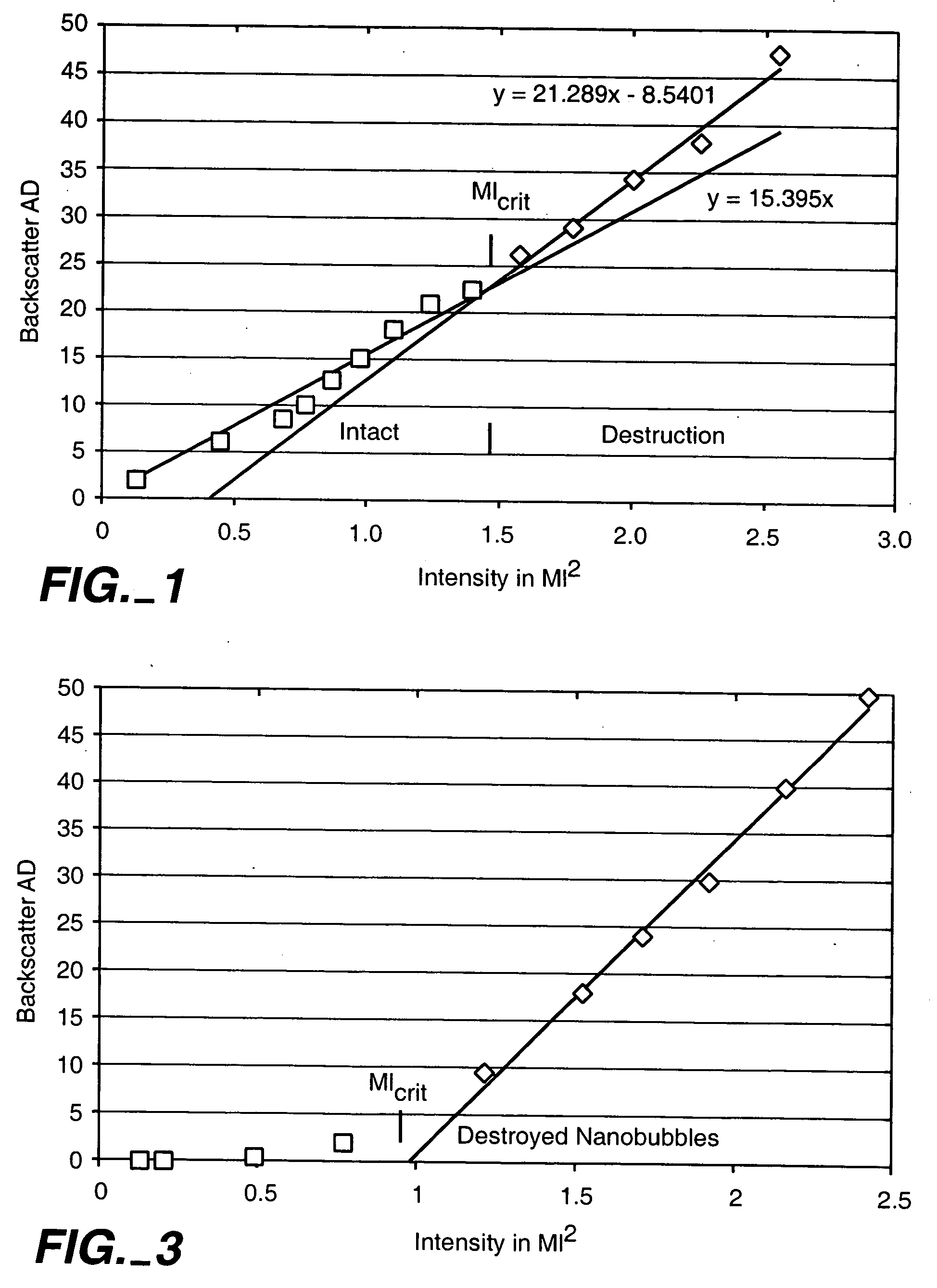
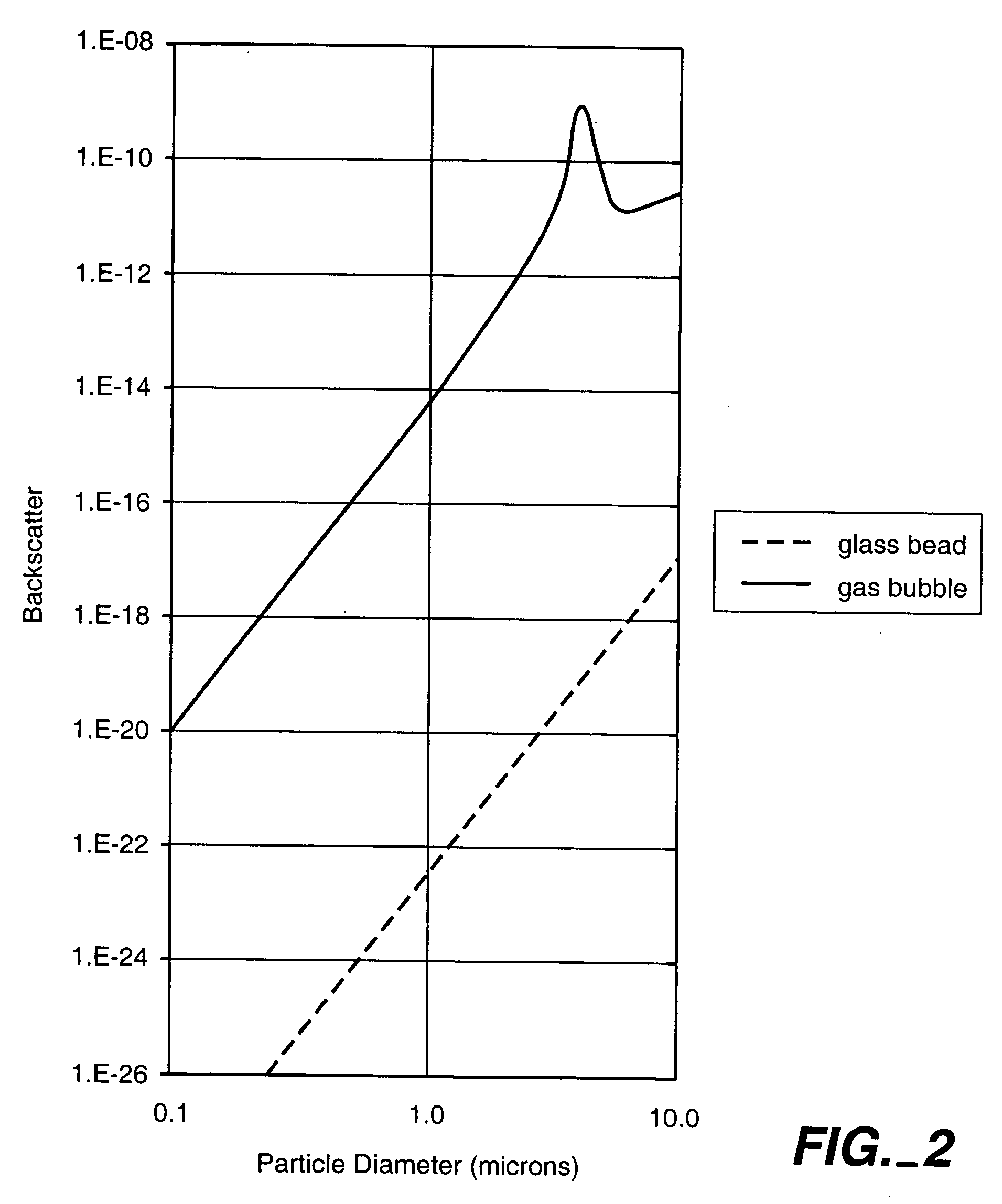
[0059] Nanobubbles fashioned in accordance with the procedures of Example 2 were tested for acoustic backscatter. For the study, an open loop flow circuit was assembled to include an ATS Laboratories Doppler flow phantom having a 6.0 mm diameter flow channel, a VWR variable flow mini-pump, and a 500 ml beaker positioned on a magnetic stir plate to serve as the reservoir for the nanobubble suspension. Flow through the phantom was adjusted to a rate of approximately 95 ml / min.
[0060] The backscatter measurements were made using an ATL HDI 5000 ultrasound system equipped with an L7-4 linear array probe. The probe was positioned onto the flow phantom so that a longitudinal image of the flow channel could be obtained. All measurements were made in harmonic B-mode at a Mechanical Index of 1.0 and focused to a depth of 2.9 cm. For each test run, a total of 30 images were taken at a triggering rate of 30 pulses per minute and then digitally stored.
[0061] A vial of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com