Devices and methods for enhanced microneedle penetration of biological barriers
a technology of enhanced microneedle and biological barrier, which is applied in the field of devices and methods for enhanced microneedle penetration of biological barrier, can solve the problems of drug not being effectively delivered in this manner, drug not being effectively diffused across the intestinal mucosa, and patient compliance may also be a problem, so as to improve interaction, enhance the transport of molecules, and limit the effect of elasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fabrication of Solid Silicon Microneedles
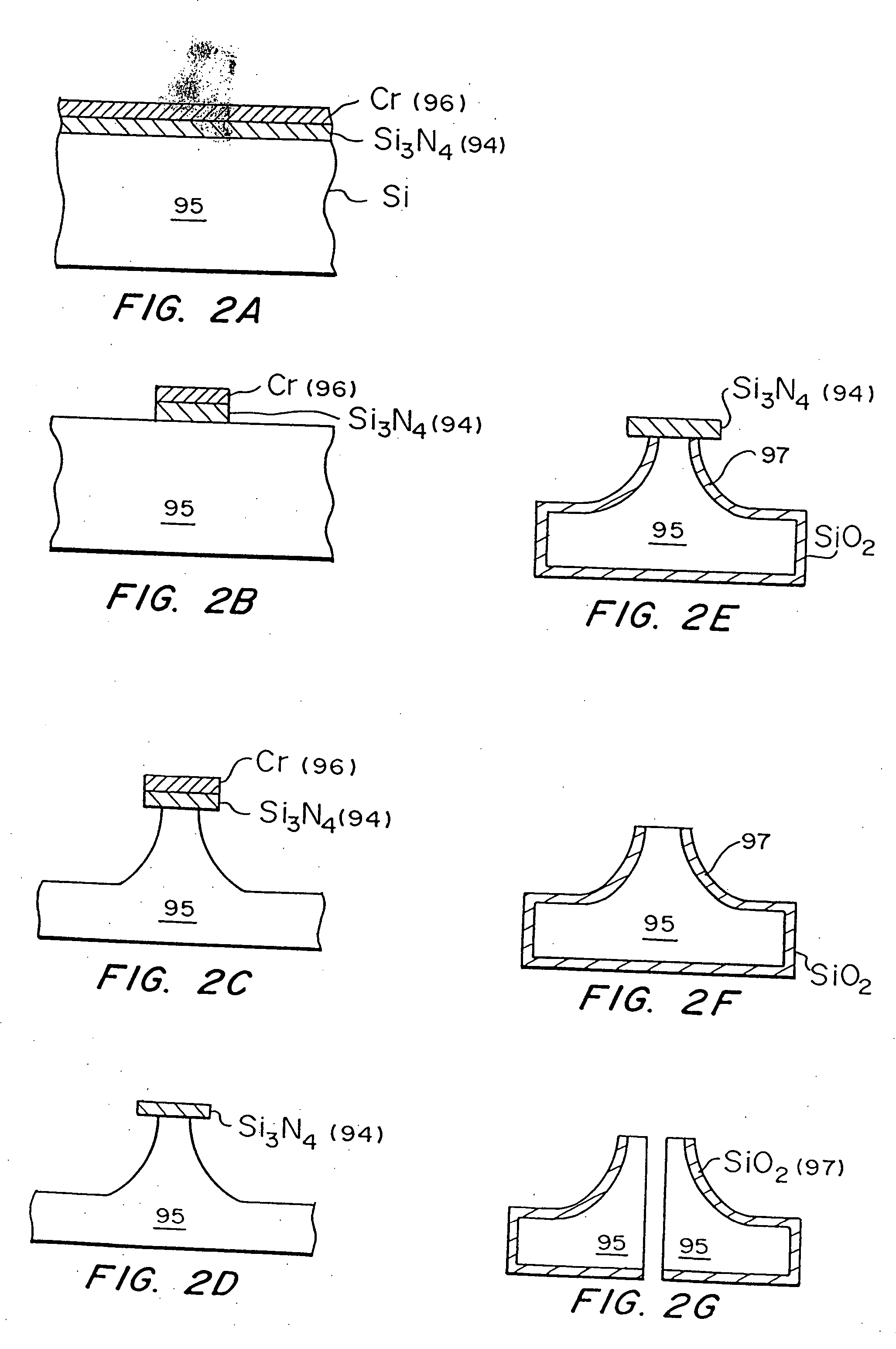
[0198] A chromium masking material was deposited onto silicon wafers and patterned into dots having a diameter approximately equal to the base of the desired microneedles. The wafers were then loaded into a reactive ion etcher and subjected to a carefully controlled plasma based on fluorine / oxygen chemistries to etch very deep, high aspect ratio valleys into the silicon. Those regions protected by the metal mask remain and form the microneedles.
[0199]-oriented, prime grade, 450-550 μm thick, 10-15 Ω-cm silicon wafers (Nova Electronic Materials Inc., Richardson, Tex.) were used as the starting material. The wafers were cleaned in a solution of 5 parts by volume deionized water, 1 part 30% hydrogen peroxide, and 1 part 30% ammonium hydroxide (J. T. Baker, Phillipsburg, N.J.) at approximately 80° C. for 15 minutes, and then dried in an oven (Blue M Electric, Watertown, Wis.) at 150° C. for 10 minutes. Approximately 1000 Å of chromium (Mat-Vac ...
example 2
Transdermal Transport Using Solid Microneedles
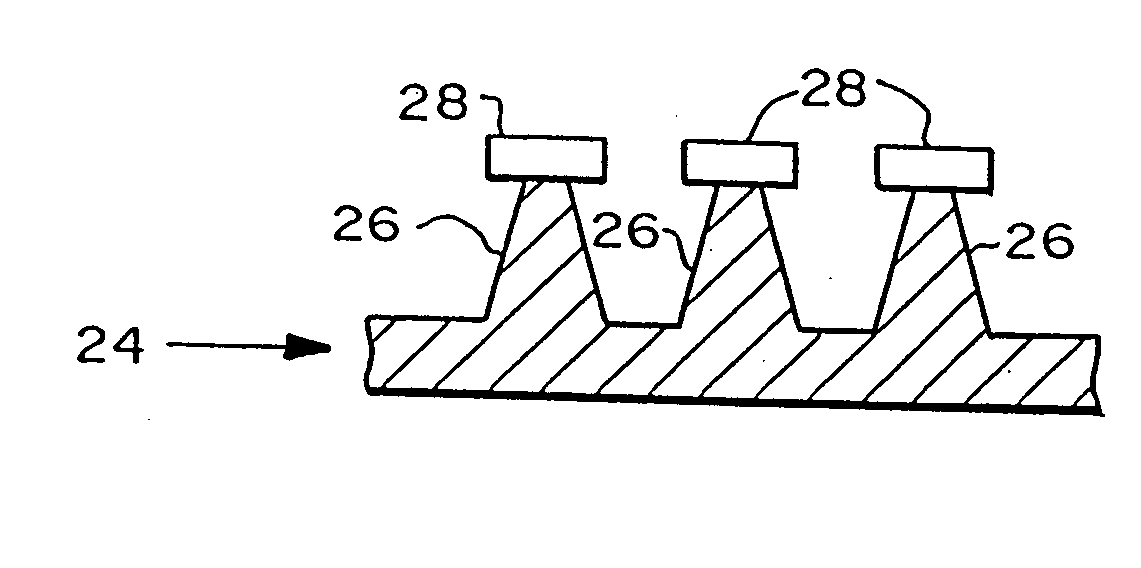
[0202] To determine if microfabricated microneedles could be used to enhance transdermal drug delivery, arrays of microneedles were made using a deep plasma etching technique. Their ability to penetrate human skin without breaking was tested and the resulting changes in transdermal transport were measured.
[0203] Arrays of microneedles were fabricated having extremely sharp tips (radius of curvature less than 1 μm), and are approximately 150 μm long. Because the skin surface is not flat due to dermatoglyphics and hair, the full length of these microneedles will not penetrate the skin. All experiments were performed at room temperature (23±2° C.).
[0204] The ability of the microneedles to pierce skin without breaking was then tested. Insertion of the arrays into skin required only gentle pushing. Inspection by light and electron microscopy showed that more than 95% of microneedles within an array pierced across the stratum corneum of the...
example 3
Fabrication of Silicon Microtubes
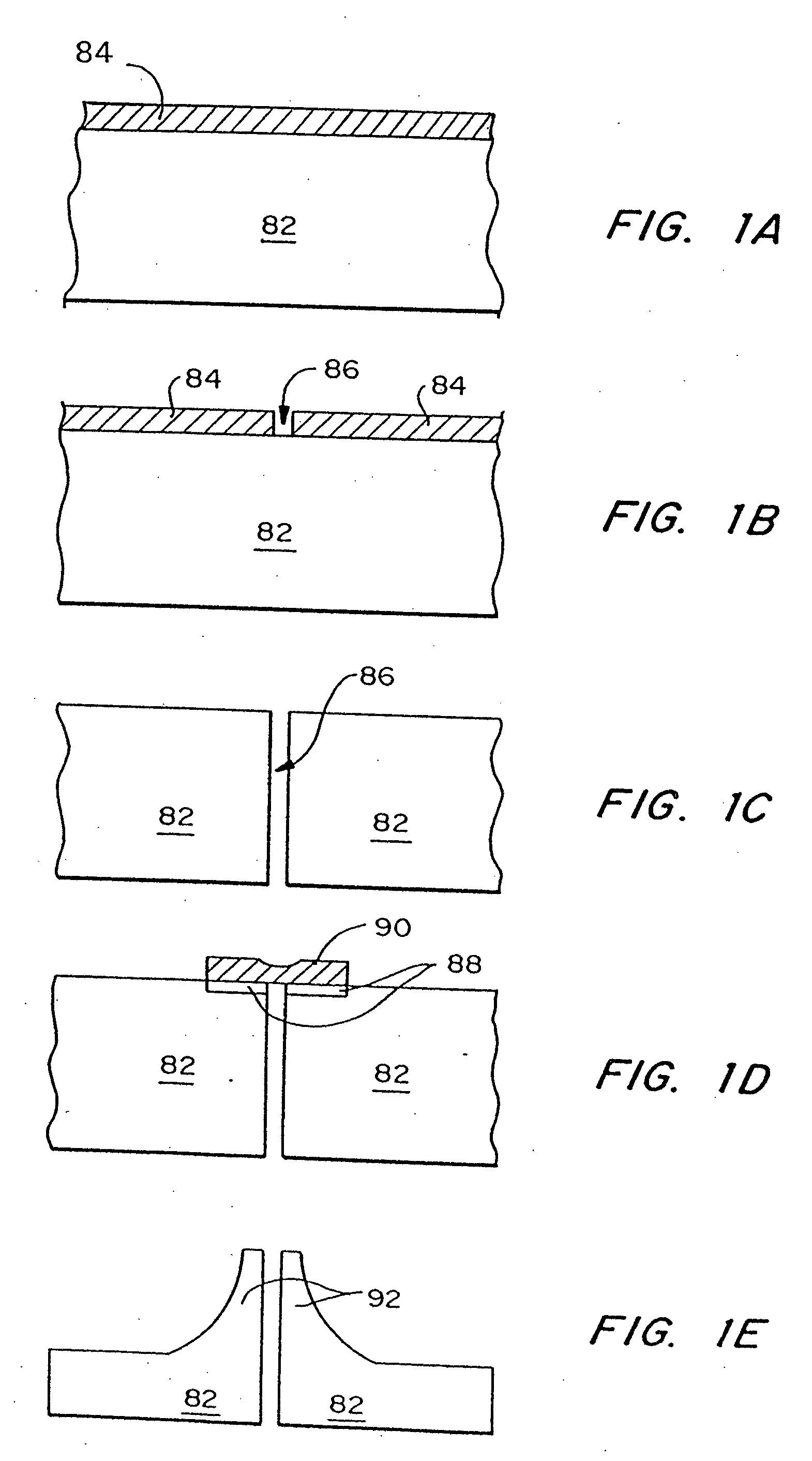
[0208] Three-dimensional arrays of microtubes were fabricated from silicon, using deep reactive ion etching combined with a modified black silicon process in a conventional reactive ion etcher. The fabrication process is illustrated in FIGS. 4a-d. First, arrays of 40 μm diameter circular holes 32 were patterned through photoresist 34 into a 1 μm thick SiO2 layer 36 on a two inch silicon wafer 38 (FIG. 4a). The wafer 38 was then etched using deep reactive ion etching (DRIE) (Laermer, et al., “Bosch Deep Silicon Etching: Improving Uniformity and Etch Rate for Advanced MEMS Applications,”Micro Electro Mechanical Systems, Orlando, Fla., USA (Jan. 17-21, 1999)). in an inductively coupled plasma (ICP) reactor to etch deep vertical holes 40. The deep silicon etch was stopped after the holes 40 are approximately 200 μm deep into the silicon substrate 38 (FIG. 4b) and the photoresist 34 was removed. A second photolithography step patterned the remaining SiO2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com