Carbapenem compound crystals and injection preparations
a technology of compound crystals and crystals, which is applied in the field of compound crystals and injection preparations of carbapenem, can solve the problems of increasing production costs, amorphous and chemically unstable carbapenem compound or a salt thereof formed by freeze-drying into a powdery charged preparation, and the salt thereof cannot be easily crystallized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-1
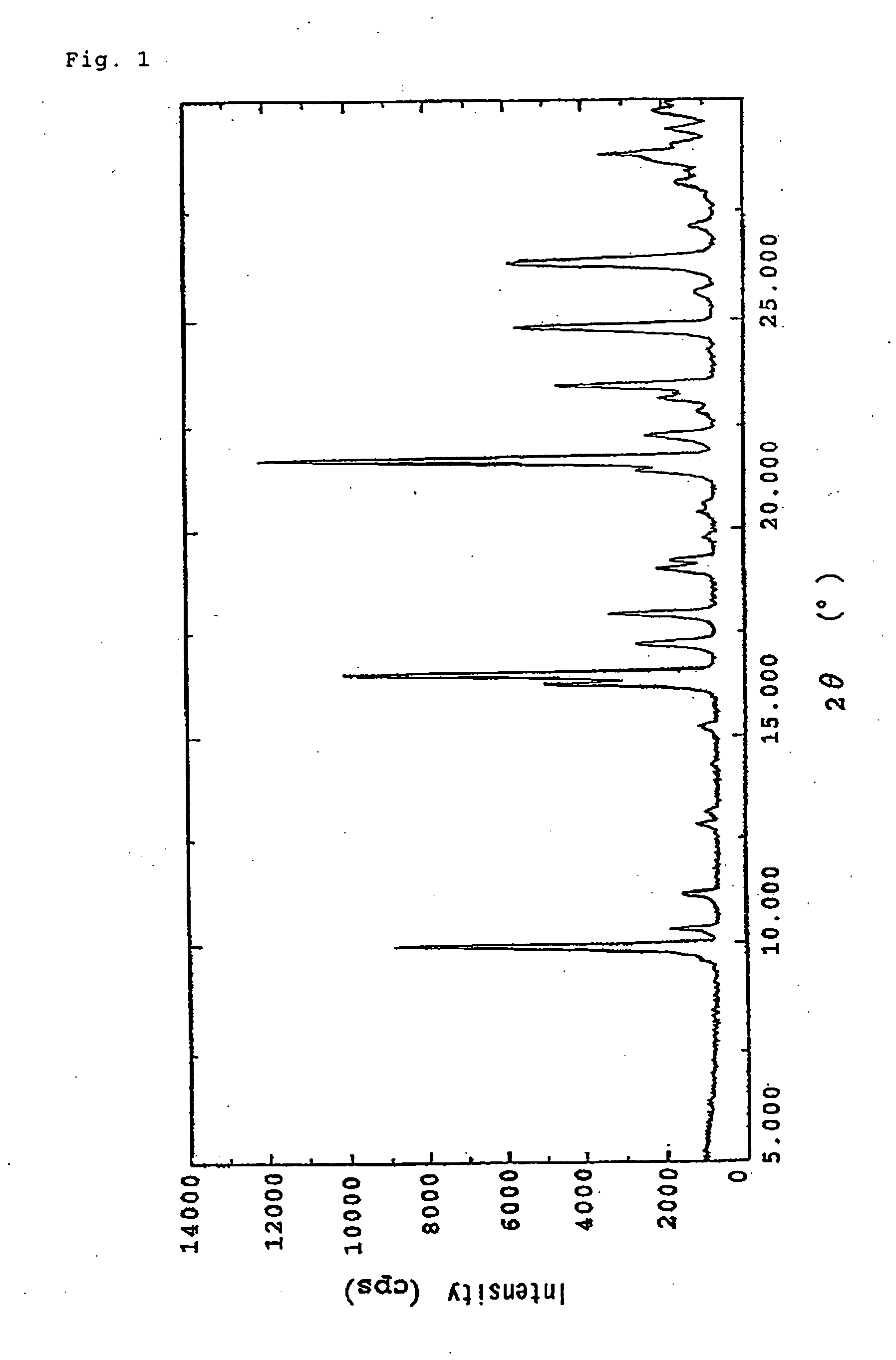
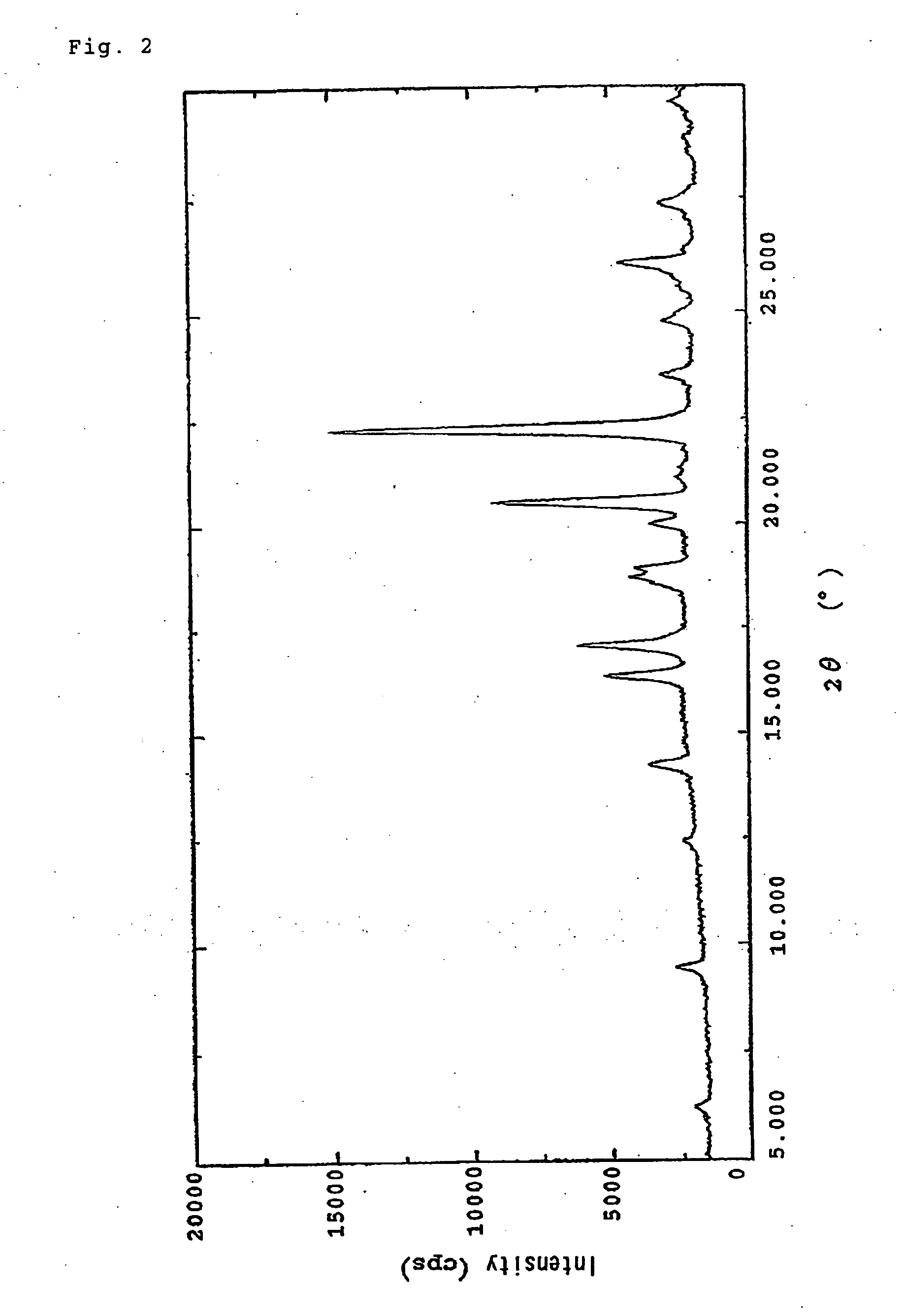
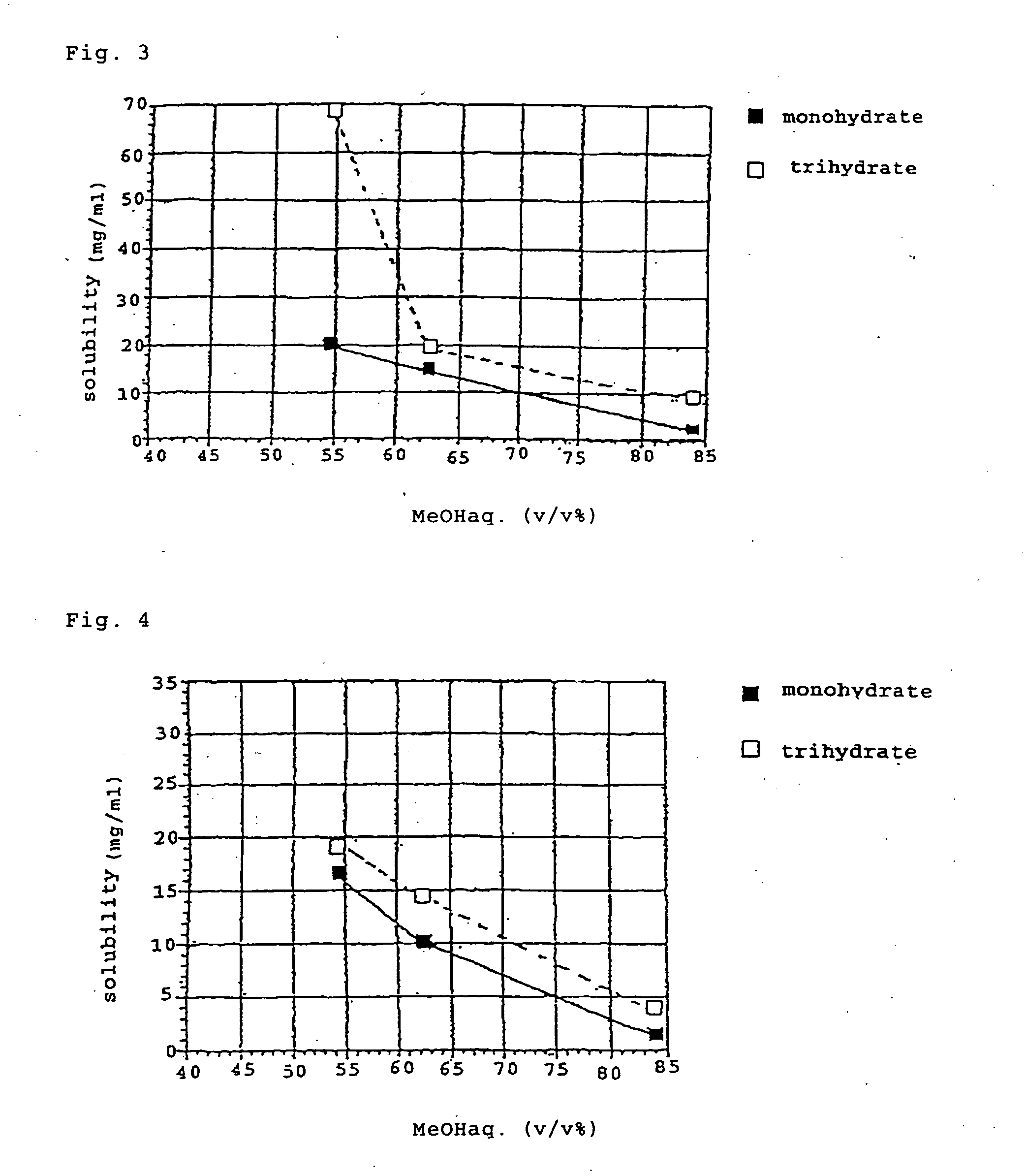
(+)-(1R,5S,6S)-6-[(R)-1-Hydroxyethyl]-1-methyl-2-[(2S,4S)-2-[(3R)-pyrrolidine-3-yl-(R)-hydroxymethyl]pyrrolidine-4-yl]thio-1-carbapen-2-em-3-carboxylic acid monohydrochloride trihydrate
[0095] Reductive de-protection of p-nitrobenzyl (1R,5S,6S)-6-[(R)-1-hydroxyethyl]-1-methyl-2-[(2S,4S)-2-[(3R)-pyrrolidine-3-yl-(R)-hydroxymethyl]pyrrolidine-4-yl]thio-1-carbapen-2-em-3-carboxylate monoxalate (29.0 g; free form, 23.11 g, 42.3 mmol) was carried out in 2 steps as follows.
[0096] p-Nitrobenzyl (1R,5S,6S)-6-[(R)-1-hydroxyethyl]-1-methyl-2-[(2S,4S)-2-[(3R)-pyrrolidine-3-yl-(R)-hydroxymethyl]pyrrolidine-4-yl]thio-1-carbapen-2-em-3-carboxylate monoxalate (14.5 g), 20% palladium hydroxide-carbon (3.08 g, 50% wet material) and H2O (333.5 mL) were introduced into a 500 mL four-necked flask equipped with a pH stat and a stirrer, and then suspended and stirred under cooling on a water bath (10° C.). After replacement by nitrogen was conducted 3 times, the mixture was vigorously stirred for 2.5 ho...
example 1-2
(+)-(1R,5S,6S)-6-[(R)-1-Hydroxyethyl]-1-methyl-2-[(2S,4S)-2-[(3R)-pyrrolidine-3-yl-(R)-hydroxymethyl]pyrrolidine-4-yl]thio-1-carbapen-2-em-3-carboxylic acid monohydrochloride trihydrate (conversion of monohydrate to trihydrate)
[0101] 1500 g aqueous solution of 174.4 g (free form, 150 g) of (+)-(1R,5S,6S)-6-[(R)-1-hydroxyethyl]]-1-methyl-2-[(2S,4S)-2-[(3R)-pyrrolidine-3-yl-(R)-hydroxymethyl]pyrrolidine-4-yl]thio-1-carbapen-2-em-3-carboxylic acid monohydrochloride monohydrate was introduced into a 10 L four-necked flask, and 1766 g of 2-propanol was added dropwise over 1 hour to this solution under stirring and cooling at 10° C. After it was confirmed that precipitation of crystals was initiated, the sample was aged for 1 hour, and 2944 g of 2-propanol (i.e. 81.6% (v / v) aqueous IPA in a 4-fold excess amount relative to the aqueous solution) was added dropwise thereto over 1 hour. After aging for 1 hour, the precipitated crystals were collected by filtration and washed with 750 mL of ...
example 1-3
(+)-(1R,5S,6S)-6-[(R)-1-Hydroxyethyl]-1-methyl-2-[(2S,4S)-2-[(3R)-pyrrolidine-3-yl-(R)-hydroxymethyl]pyrrolidine-4-yl]thio-1-carbapen-2-em-3-carboxylic acid monohydrochloride monohydrate
[0102] Out of the solution (996.8 g) clarified by filtration with a glass filter (GA100) in Example 1-1, 849.1 g solution (free form: 10.79 g) was adjusted to pH 8.5 with 1 N aqueous sodium hydroxide, and the solution (871.4 g) was purified by applying it onto a resin (SP850) column (5 cmΦ×50 cm, flow rate of 50 mL / min, previously equilibrated with 0.05 M phosphate buffer). The column was charged with 20% aqueous methanol solution containing 0.05 M phosphate buffer, water and 1.0 equivalent of hydrochloric acid and then with 20% (v / v) aqueous methanol solution, and the resulting major fractions were stored overnight at 10° C. or less (yield, 81%). Out of the resulting solution (1985 g, containing 8.74 g free compound), 1621.1 g solution was concentrated into 125.2 g concentrate (containing 7.08 g fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com