Phytase enzymes, nucleic acids encoding phytase enzymes and vectors and host cells incorporating same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evidence of Phytate Hydrolysing Activity in Liquid Culture
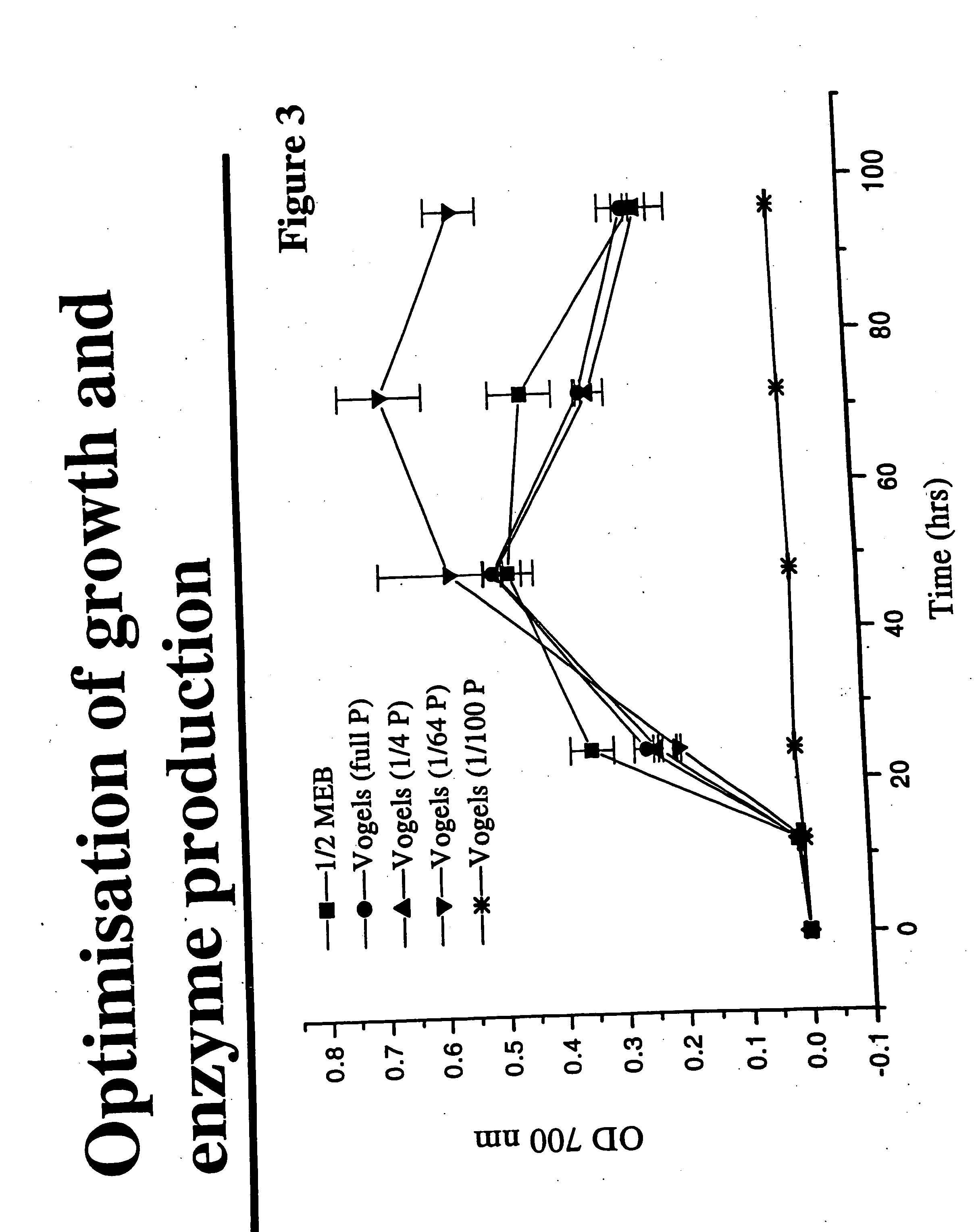
[0191]P. piceum (ATCC No. 10519) and P. hordei (ATCC No. 22053) were grown in defined media containing various concentrations of inorganic phosphate, and growth characteristics and phytase production were assayed and compared. Spore suspensions were used (2×106 spores / ml final con) to inoculate a minimal media (Vogels) where the phosphate concentration was altered to see how this would affect growth and phytase production. Cultures were grown in 50 ml of medium in shake flask culture at 25° C. (P. hordei) or 30° C. (P. piceum). Cultures were harvested at 24, 48, 72 and 96 hours. Cultures supernatants were assayed for phytase activity using the method of Fiske and SubbaRow. Growth was determined by dry weight (P. hordei) or OD readings (P. piceum).
1A. Effect of Different Media Conditions on Growth and Morphology
[0192] The series of Penicillium growth curves that were produced, such as the P. piceum growth curve of FIG. 3 a...
example 2
PCR Amplification of Phytase Gene Fragments
2A. Degenerate Primer Design
[0209] Based on alignments of published phytase amino acid sequences, a range of degenerate primers were designed against conserved structural and catalytic regions. Such regions included those that were highly conserved among the phytases, as well as those known to be important for enzyme structure and function.
[0210] In one study, amino acid sequences for 4 published phytases were aligned. The sequences were of phytases from: (i) A. niger (DEFINITION: A. niger phyA gene.; ACCESSION: Z16414; van Hartingsveldt, W., et al, 1992); (ii) A. fumigatus (DEFINITION: Aspergillus fumigatus phytase gene, complete cds.; ACCESSION: U59804; Pasamontes, L., et al, 1997); (iii) A. terreus (DEFINITION: Aspergillus terreus 9A1 phytase gene, complete cds.; ACCESSION: U59805; Mitchell, D. B., et al, 1997); and (iv) Myceliophtora thermophilus (DEFINITION: Myceliophthora thermophila phytase gene, complete cds.; ACCESSION: U59806;...
example 3
Southern Analysis for Library Production
[0223] Genomic DNA from P. hordei, P. piceum and A. niger were digested with a range of restriction enzymes overnight at 37° C. Successfully digested DNA was run out on a 1% agarose gel in preparation for transfer to the nylon membrane. After completion of electrophoresis, the agarose gel was soaked for 10 min. in 0.2M HCl to depurinate the DNA and then rinsed briefly in ddH2O. The DNA was transferred to the Hybond™-N+ membrane (Amersham International PLC) by alkali capillary blotting. The blot was set up so that the nylon filter was sandwiched between the gel and a stack of absorbent paper towels. A wick of Whatman 3MM paper (Schleicher and Schuell, Dassel, Germany) was prepared on a glass plate over a reservoir of transfer buffer (0.4M NaOH). The gel was inverted on the wick, taking care to avoid the formation of air bubbles, and surrounded by strips of Nescofilm to prevent the blotting action of the paper towels from by-passing the gel at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com