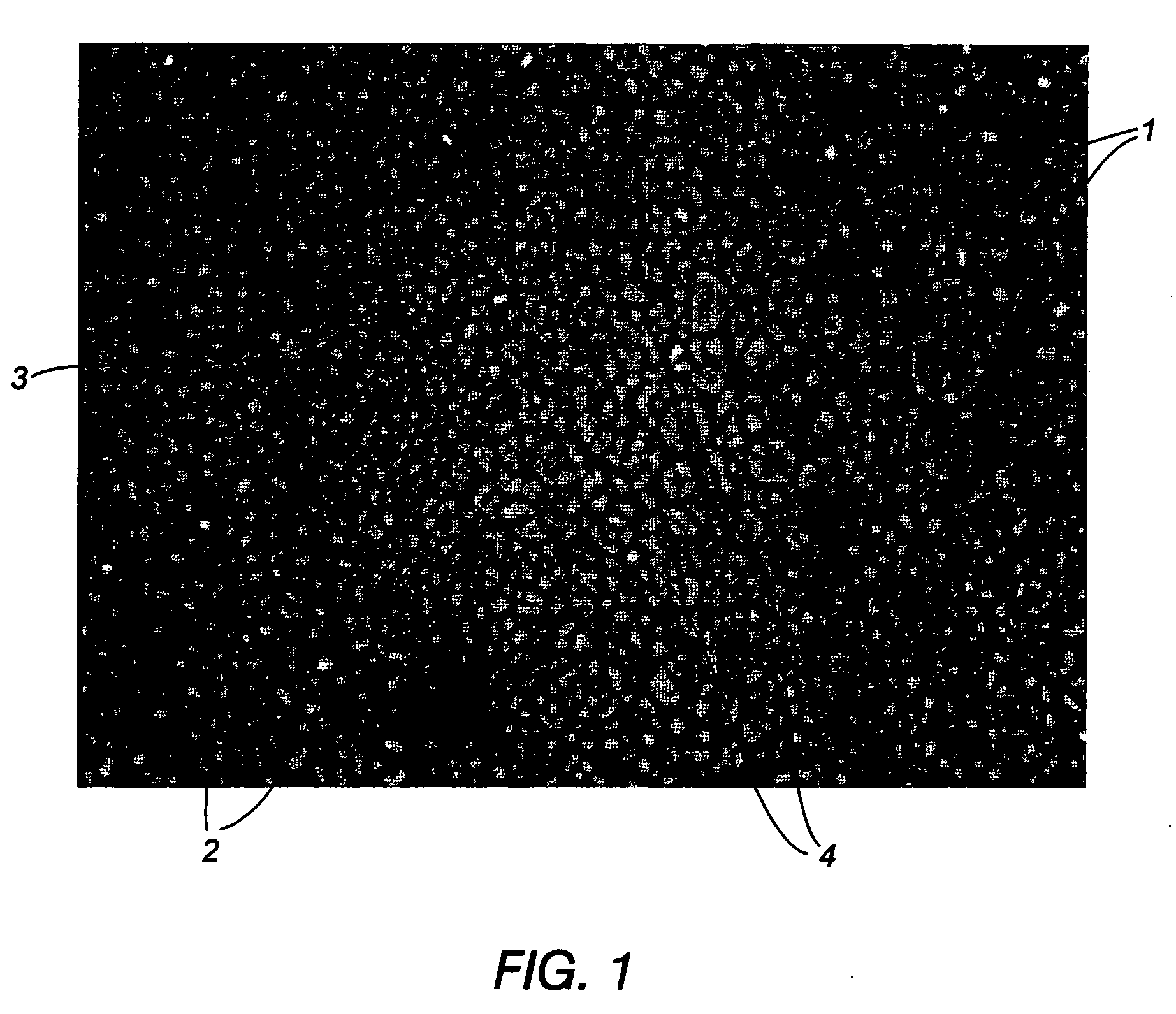
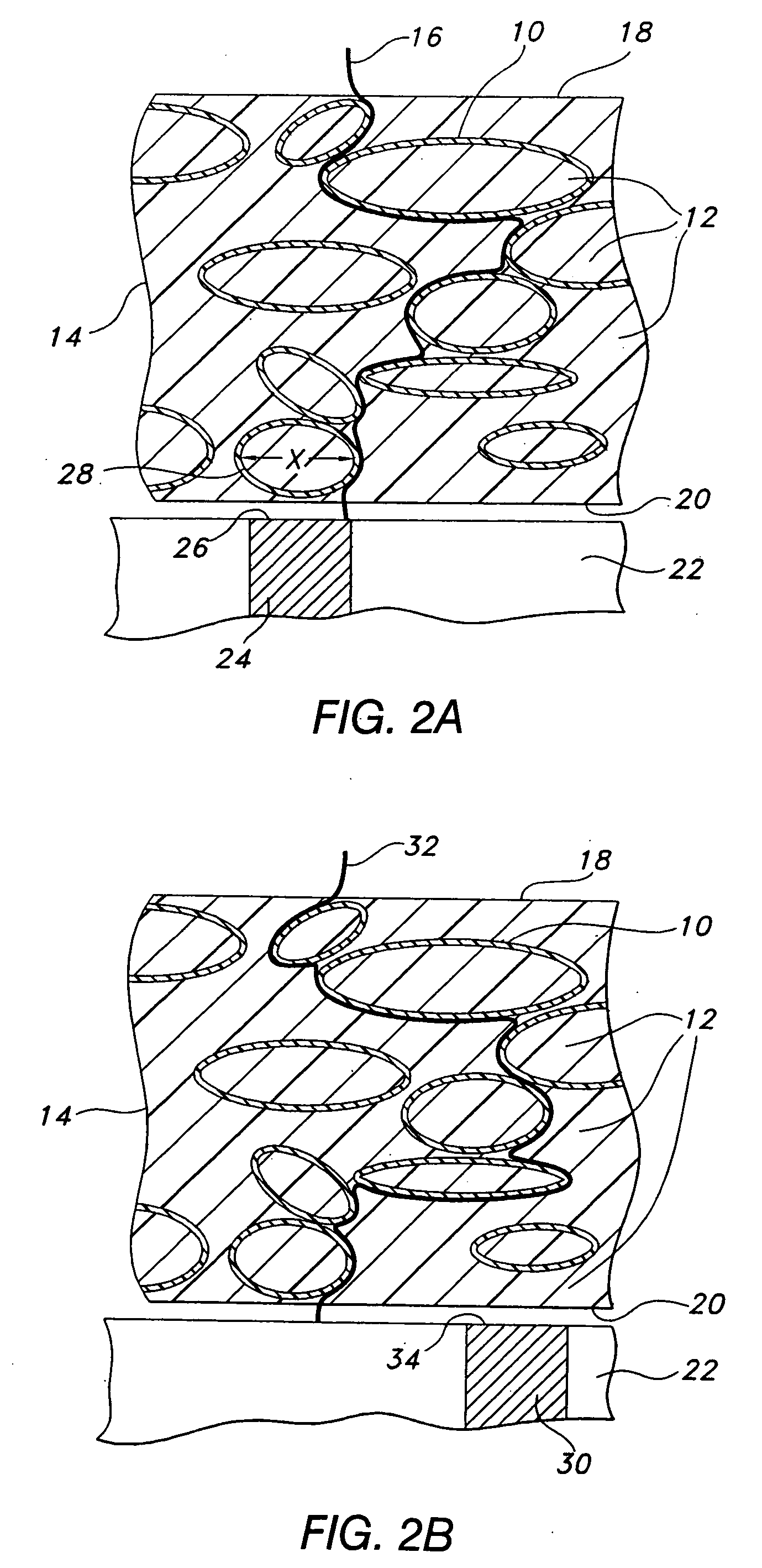
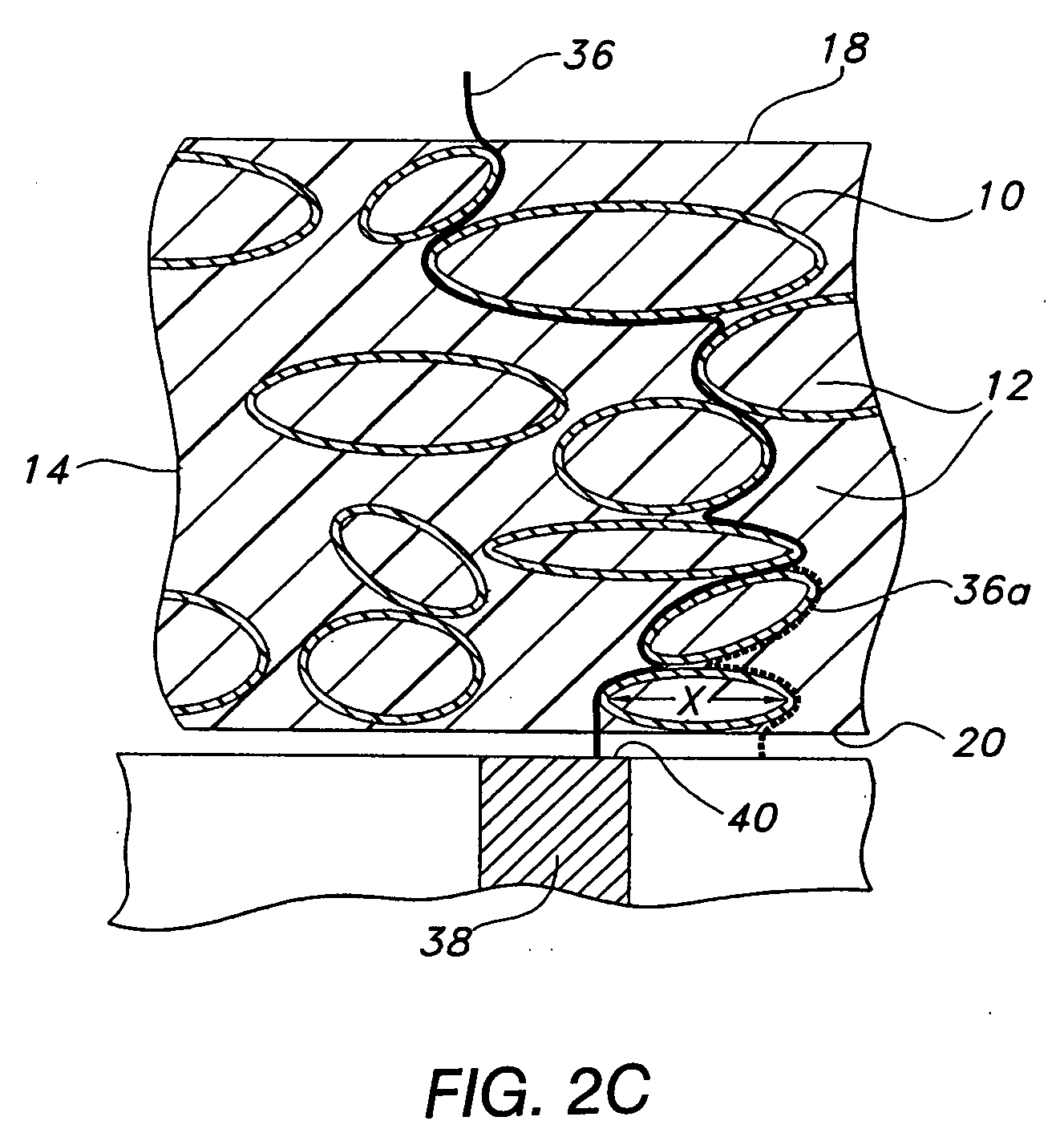
Techniques to improve polyurethane membranes for implantable glucose sensors
a technology of glucose sensor and polyurethane, which is applied in the direction of sensors, pretreated surfaces, synthetic resin layered products, etc., can solve the problems of reducing the ease with which the membrane may be manufactured, the oxygen deficit is not easily achieved, and the re-usability of the membrane is not easy to achieve. achieve the effect of reproducibility and easy fabrication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Method for Preparing a Membrane of the Present Invention
[0079] The inventive membrane may be cast from a coating solution. The coating solution is prepared by placing approximately 281 gm of dimethylacetamide (DMAC) into a 3 L stainless steel bowl to which a solution of polyetherurethaneurea (344 gm of Chronothane H (Cardiotech International, Inc., Woburn, Mass.), 29,750 cp @ 25% solids in DMAC) is added. To this mixture is added another polyetherurethaneurea (approximately 312 gm, Chronothane 1020 (Cardiotech International, Inc., Woburn, Mass.), 6275 cp @ 25% solids in DMAC). The bowl is then fitted to a planetary mixer with a paddle-type blade and the contents are stirred for 30 minutes at room temperature. Coatings solutions prepared in this manner are then coated at between room temperature to about 70° C. onto a PET release liner (Douglas Hansen Co., Inc., Minneapolis, Mn.) using a knife-over-roll set at a 0.012 inch gap. The film is continuously dried at 120° C. to about 1...
example 2
Optimizing the Coating Solution Conditions
[0082] This example demonstrates that preheating the coating solution to a temperature of 70° C. prior to coating eliminates the presence of both the circular and elliptical domains that were present throughout the hydrated cross-section of a membrane prepared using a room temperature coating solution and drying of the coated film at 120° C. Example 2 further demonstrates that, provided the coating solution is preheated to about 70° C., either a standard (120°) or elevated (150° C.) drying temperature were sufficient to drive the DMAC solvent from the coated film quickly to further inhibit the hydrophilic and hydrophobic portions of the polyurethane membrane from segregating into large domains.
[0083] In particular, the invention was evaluated by performing a coating experiment where standard coating conditions (room temperature coating solution and 120° C. drying temperature of the coated film) were compared to conditions where the coatin...
example 3
Evaluation of The Inventive Membranes for Their Permeability to Glucose and H2O2
[0089] Membranes prepared under the EE condition described in Example 2 were evaluated for their ability to allow glucose and hydrogen peroxide to get through the membrane to a sensor. In particular, a series of polyurethane blends of the present invention were generated wherein the percentage of Chronothane H in a coating blend was varied. Furthermore, one of these blends (57.5% Chronothane H in coating blend) was prepared under both the EE condition and the SS condition as described in Example 2. FIG. 6 shows that the sensor output generated with a series of polyurethane blends of the present invention was dependent upon the percentage of the Chronothane H. In particular, the sensor output increased as the percentage of Chronothane H in the coating blend increased. With further reference to FIG. 6, when the percentage of Chronothane H in the coating blend was 57.5%, the sensor output was three times ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com