Methods and compositions for promoting immunopotentiation
a technology of immunopotentiation and composition, applied in the field of immunopotentiation, can solve the problems of imposing severe restrictions on triggering an immune response, too late for the immune system to save the organism, and too low doses of invading antigens to trigger an immune response, etc., to achieve rapid rise in intracellular calcium, increase cell proliferation, and increase the effect of il-2 receptor expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Immunopotentiating Agents
1. Single Agents
[0128] A. Monoclonal Antibodies
[0129] Monoclonal antibodies were prepared against specific classes of T cell epitopes. These classes are listed in Table 3. In the following embodiment, methods for preparing a monoclonal antibody against a nonpolymorphic epitope, CD3, and polymorphic epitope Vβx where x=a specific chain in the variable part of the TcR complex, are described:
[0130] (1) Preparation of a mAb Directed Against the Murine CD-3 Chain of the TcR Complex
[0131] Monoclonal antibodies were generated which are reactive with the T cell surface structures expressed as alloreactive cytotoxic T cell (CTL) clones and involved in T cell activation. MAbs specific for these cell surface molecules were identified using an assay (developed by Bluestone, the redirected lysis assay (33), see also Example 13) based on the ability of antibodies reactive with the TcR complex to induce antigen-specific CTL to lyse cells which are ...
example 2
Activation of T-cells by Administration of Anti-CD3
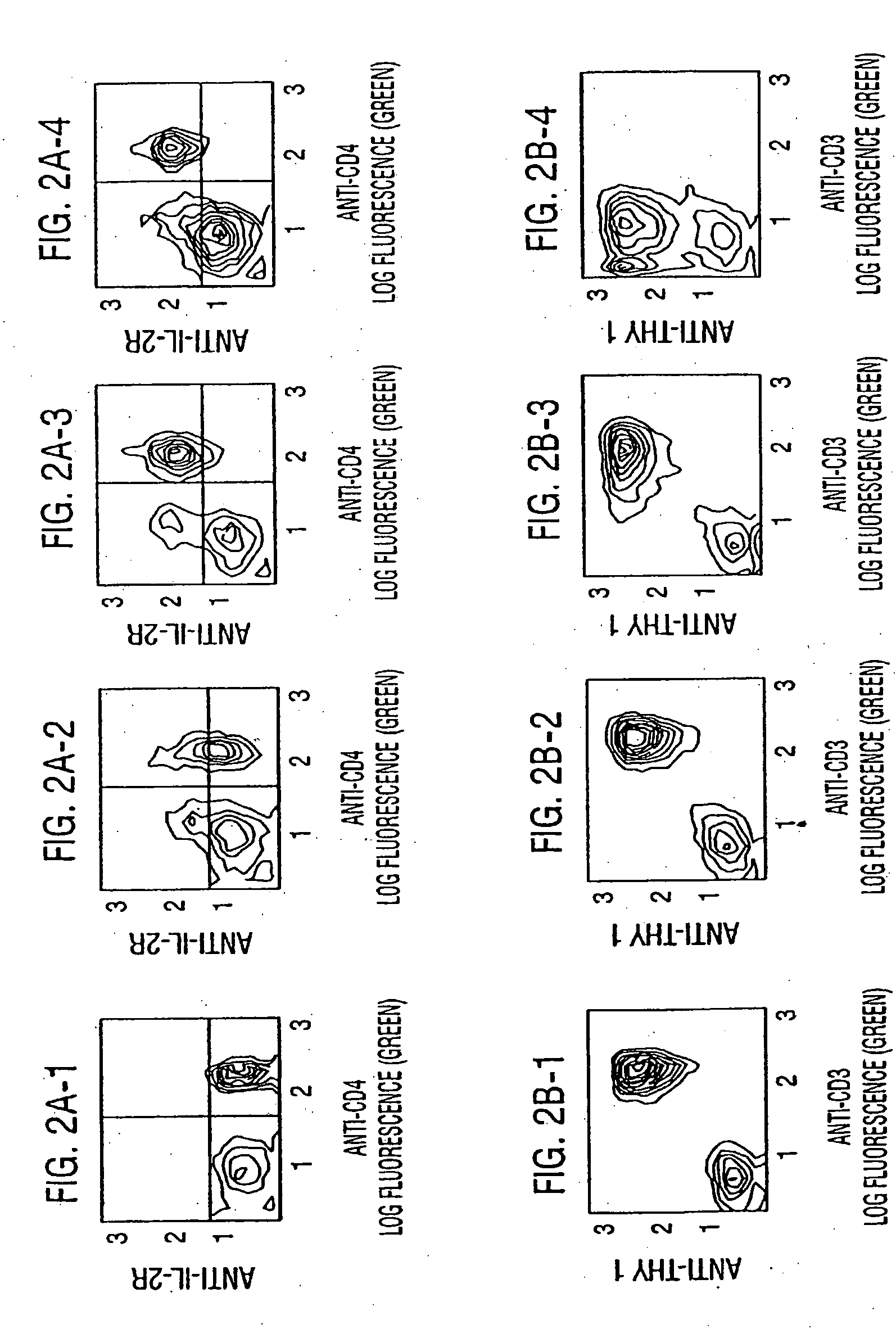
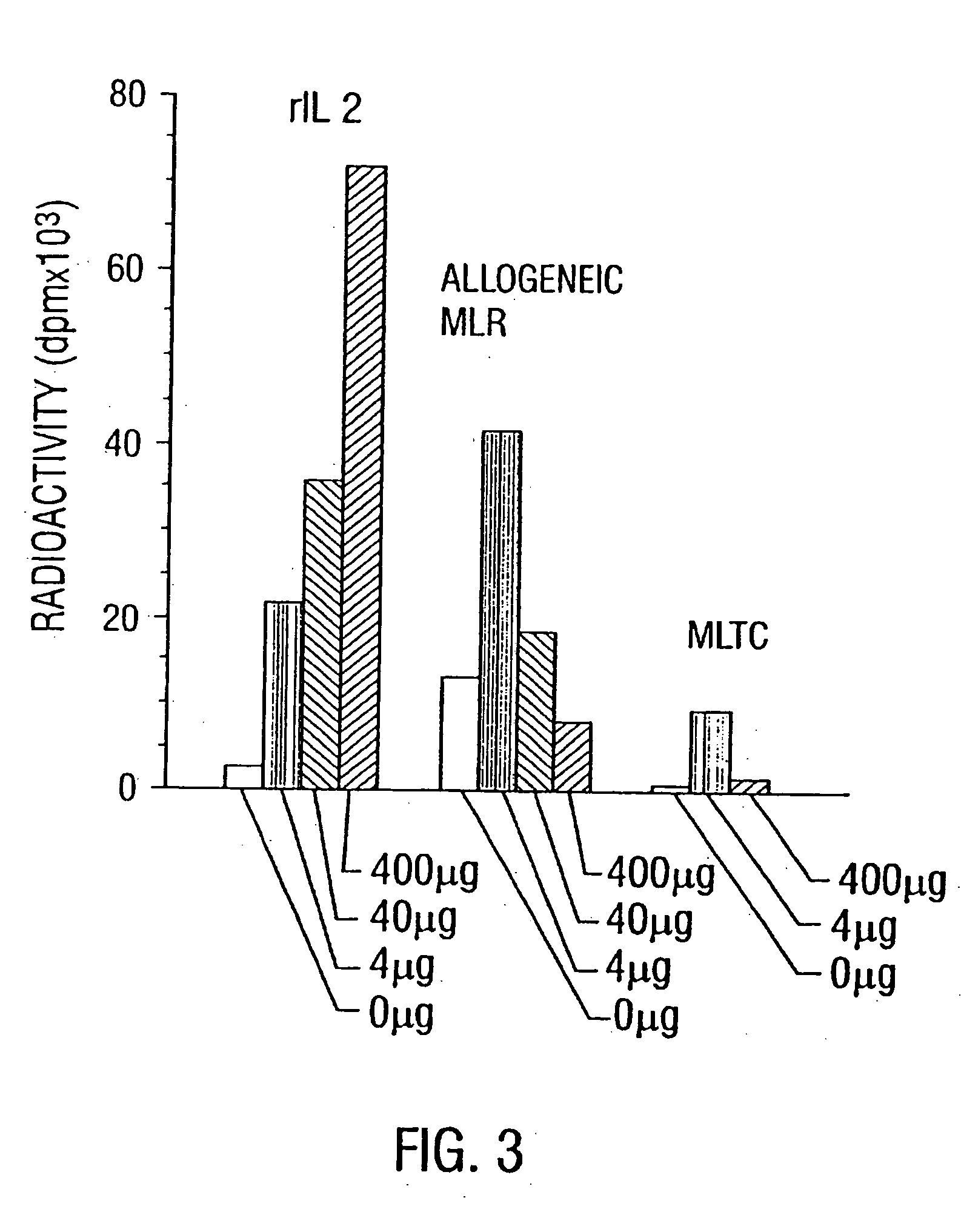
[0149] To evaluate whether low doses of anti-CD3 were effective as activating T cells in mice, mice were given different doses and their lymph nodes and spleen cells were examined for IL-2R expression by flow-cytometry. IL-2R expression was enhanced at the three doses tested (4, 40 and 400 micrograms) and plateaued at 400 micrograms. (FIG. 2). When the same lymphoid cells were incubated in media containing human rIL2, their proliferation was enhanced in proportion to their IL-2R expression. The immune suppression which results from a dose of 400 microgram of anti-CD3 was the result of T cell depletion, T cell receptor blockade and modulation of the TcR complex. The net result was that the amount of cell surface CD3 available to react with antigen was decreased, rendering the T cell unable to respond to antigenic stimuli because efficient antigenic specific activation depends on the presence of intact TcR. Therefore the quantity of ...
example 3
Effect of Anti-CD3-Treatment on Sendai Virus Infection in Mice
[0150] The purpose of this example was to test the effect of mAb treatment on infection. Administration of low doses of anti-CD3 prevented the lethal pneumonia caused by the Sendai virus in >60% of mice. Anti-CD3 treated, virally-infected mice also developed lasting virus-specific immunity as evidenced by their ability to withstand a subsequent dose of Sendai virus of 1000 times the LD50 dose. Treated mice also developed a Sendai virus specific DTH and antibody response similar to mice immunized with a non-virulent Sendai virus vaccine. Interestingly, the 129 / J strain of mice were also protected by the anti-CD3 treatment. Because virus susceptibility in these mice has been shown to be caused by an inadequate generation of NK cell responses, it appears as if NK activity induced by the mAb treatment contributes to viral protection. (51)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com