Processes and systems for formation of high voltage, anodic oxide on a valve metal anode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
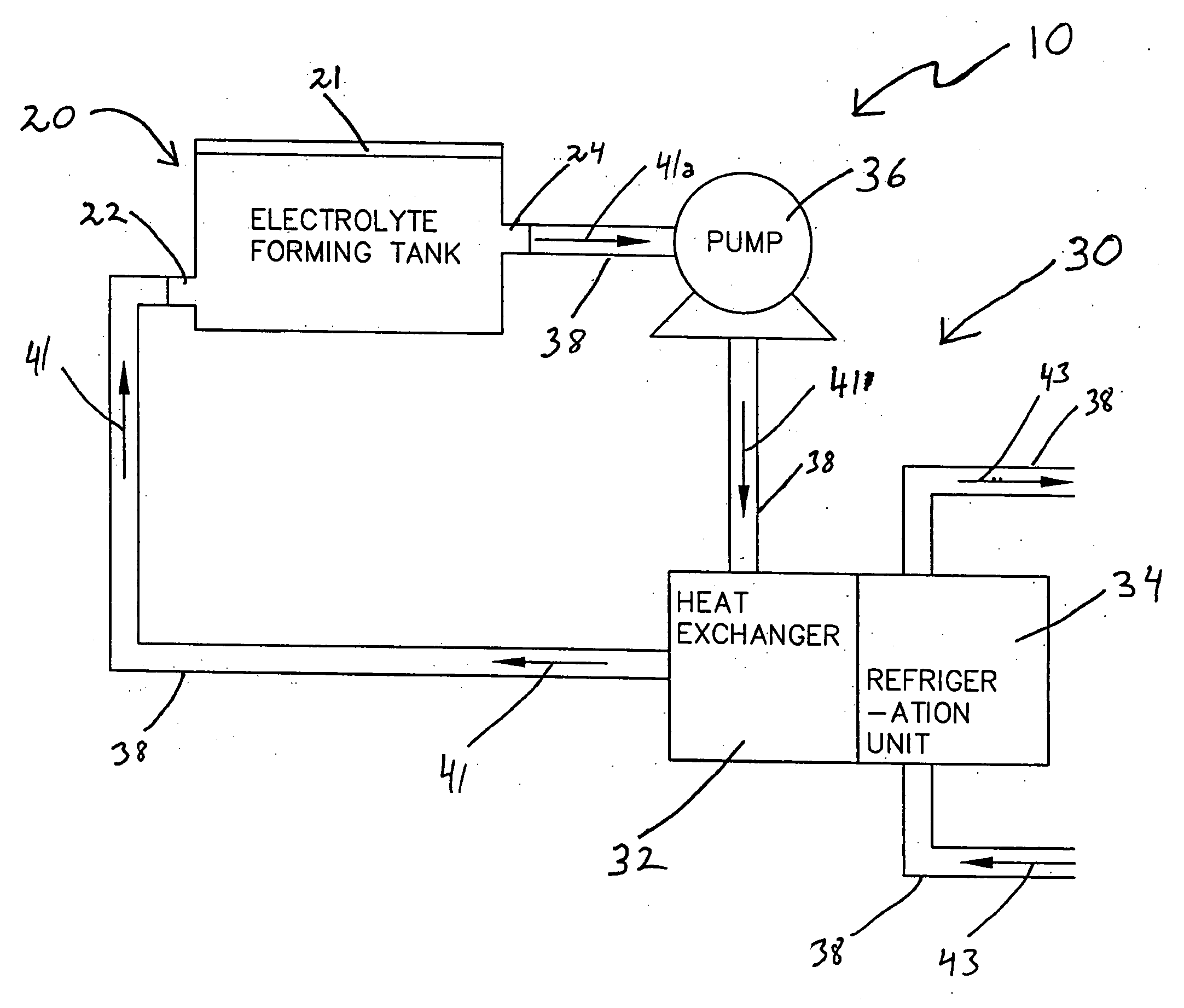
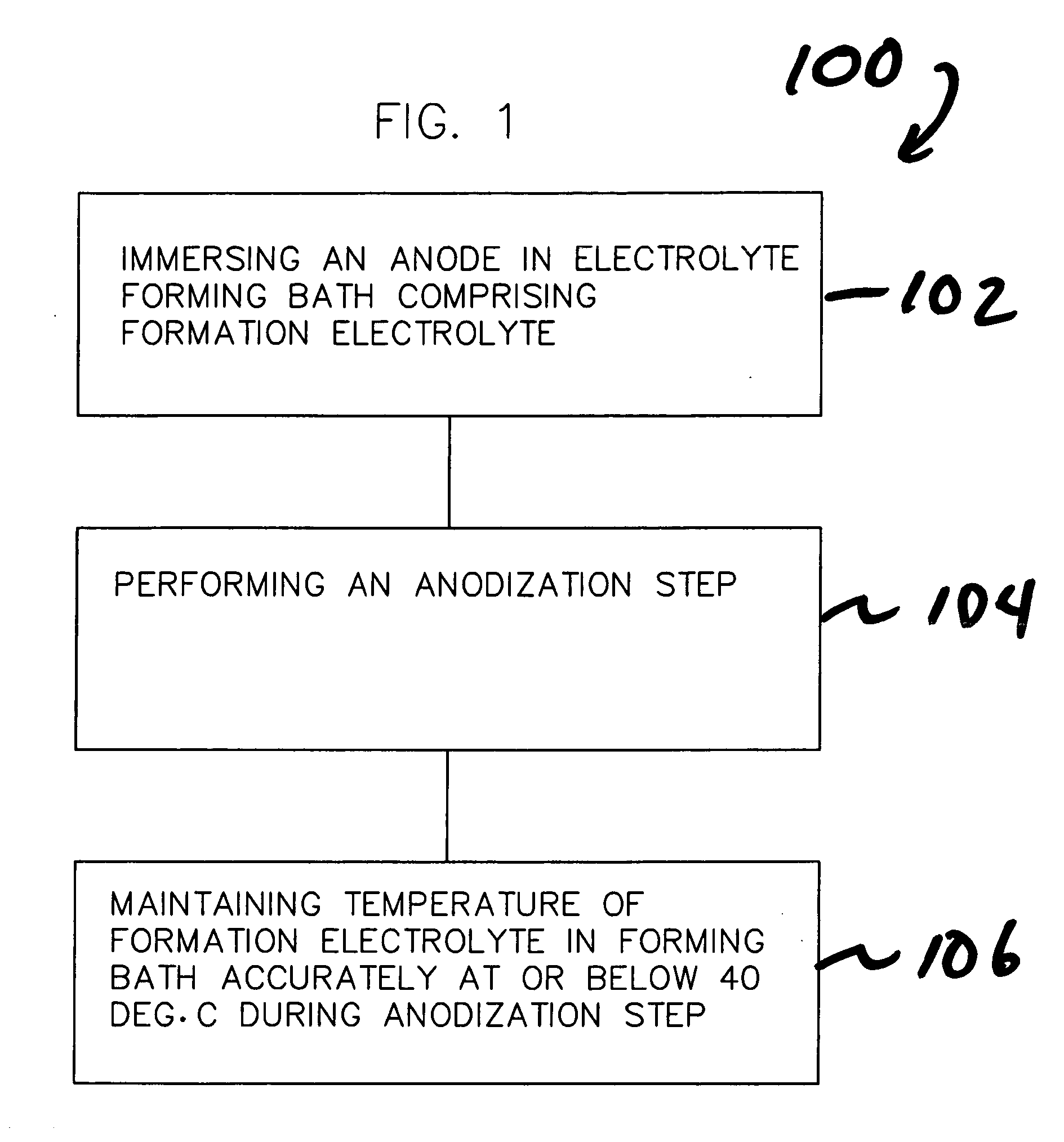
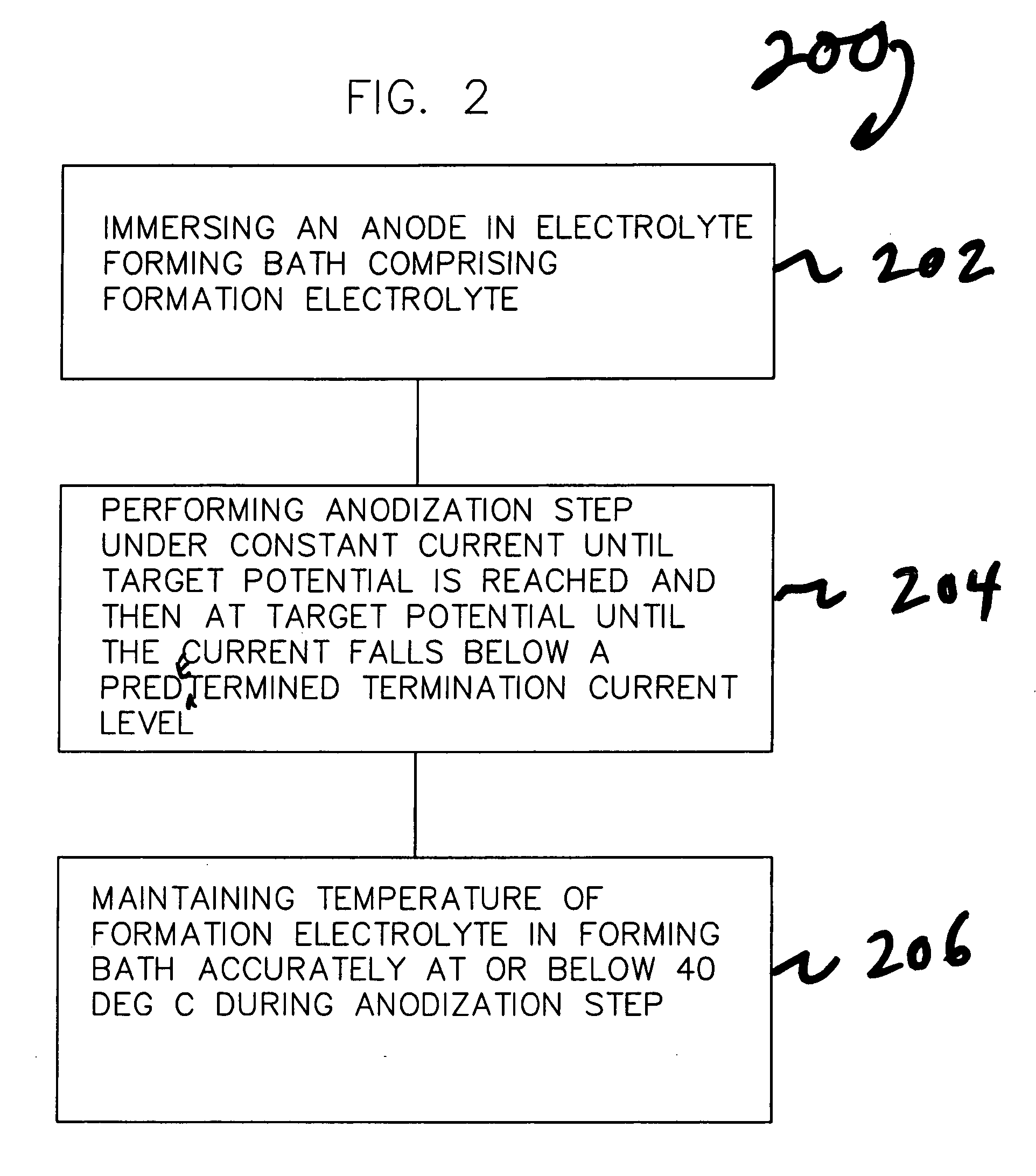
[0081] A set of 8 capacitors were formed with the pulsed formation technique depicted in FIG. 8 and discussed earlier herein. Anodes 1 through 4 were processed in a system without active temperature control, which allowed the bath temperature to fluctuate or climb up to 40° C. at the time of maximum power dissipation, a traditional method. Anodes 5 through 8 were processed in a system with accurate temperature control of the formation bath, keeping the temperature constant at 18° C., a method according to the invention. Processing conditions were otherwise the same for all 8 electrodes, with the exception of the use of active temperature control according to the invention in the processing of Anodes 5-8 to accurately maintain the temperature of the formation electrolyte at 18° C. The target potential was 260 V, initial formation current was 275 mA for four anodes. Formation frequency was about 0.2 mHz with a duty cycle between 95% and 75% depending on the power dissipation. Table 1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com