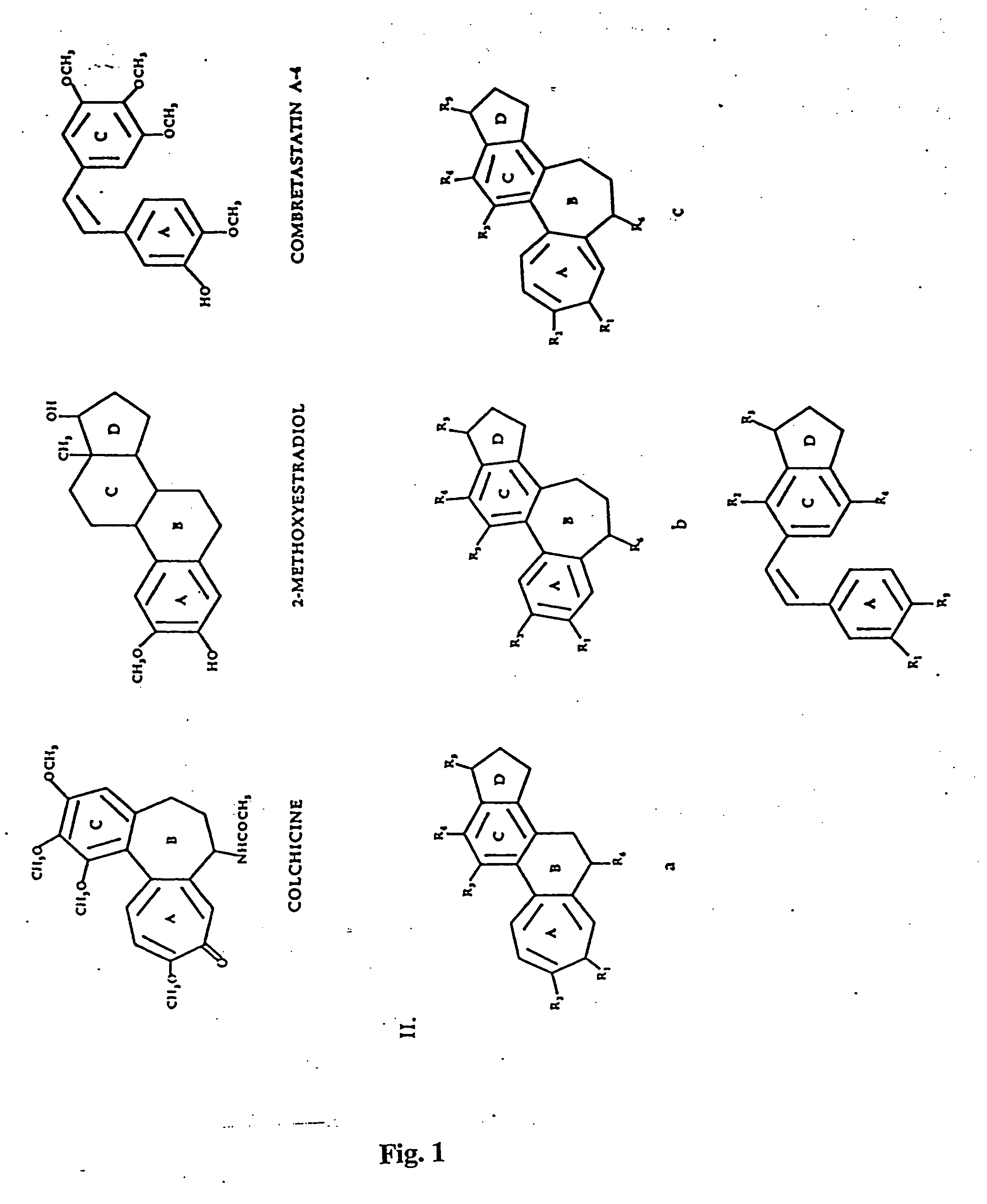
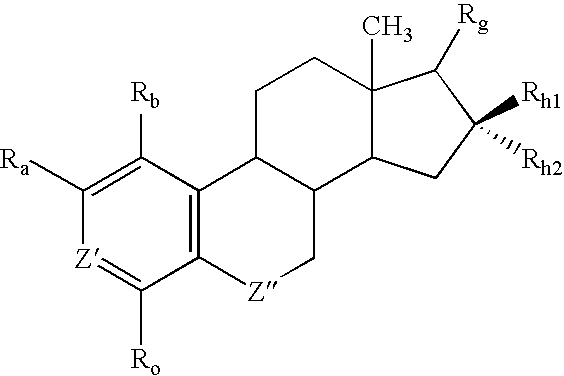
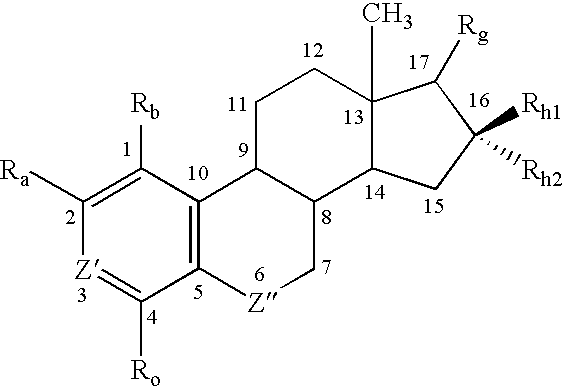
Antiangiogenic agents
an antiangiogenic agent and angiogenesis technology, applied in the field of antiangiogenic agents, can solve the problems of not being able to undergo demethylation of analogs, and achieve the effects of improving absorption, transport, biological stability, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2-ME Derivatives and Modifications at the 16 Position
[0079] Synthesis of the 2-ME derivatives described herein is within the capability of one ordinarily skilled in the art. A specific description of the synthesis of the 2-ME derivatives having modifications at the 2 and 6 positions and analogs discussed herein can be found in M. Cushman, H-M. He, J. A. Katzenellenbogen, C. M. Lin and E. Hamel, Synthesis, antitubulin and antimitotic activity, and cytotoxicity of 2-methoxyestradiol, and endogenous mammalian metabolite of estradiol that inhibits tubulin polymerization by binding to the colchicine binding site, J. Med. Chem., 38(12): 2042 (1995); and M. Cushman, H-M. He, J. Katzenellenbogen, R. Varma, E. Hamel, C. Lin, S. Ram and Y. P. Sachdeva, Synthesis of analogs of 2-methoxyestradiol with enhanced inhibitory effects on tubulin polymerization and cancer cell growth, J. Med. Chem. 40(15): 2323 (1997).
[0080] The synthetic pathways used to prepare the derivatives of the...
example 2
Preparation of 3-Benzyl-2-methoxyestradiol
[0081] 2-Methoxyestradiol (10.09 g, 33.4 nmmol) and potassium carbonate (22 g, 278 mmol) were suspended in anhydrous ethanol and cooled to 0° C. Benzyl bromide (11.4 mL, 95.8 mmol) was added dropwise, and following the addition, the mixture was brought to reflux for 8 h. The solution was cooled to room temperature (rt), and the solvent was removed via rotoevap. The resulting residue was diluted with approximately 200 ml water, and washed with ethyl acetate (3×200 mL). The combined organics were washed with water (200 mL), sodium bicarbonate (saturated (satd), 200mL) and brine (200 mL). Dry with sodium sulfate, filter and roto-evaporation (rotoevap). Product was dried under vacuo with occasional gentle heating using a heat gun to give a yellowish glass (13.54 g, quanitative yield) and used without further purification.
[0082] Selected spectral data: 1H-NMR (300 MHz, CDCl3) δ 7.29-7.53 (m, 5H), 6.88 (s, 1H), 6.65 (s, 3H), 5.11 (s, 2H), 3.87 ...
example 3
Preparation of 3-Benzyl-2-methoxyestrone
[0083] Oxalyl chloride (38 mmol, 19 mL, 2M, methylene chloride) was added to anhydrous methylene chloride (25 mL) and cooled to −46° C. Methyl sulfoxide (5.40 mL, 76 mmol) was added dropwise, and the mixture was stirred for 2 minutes. 3-Benzyl-2-methoxyestradiol in methylene chloride / methyl sulfoxide (10 mL / 15 mL) and added within 5 minutes and the resulting mixture was stirred for 1 h. Triethyl amine (170 mmol, 23.5 mL) was added drop-wise, stirred 5 minutes and warmed to rt. Water (˜200 mL) was added and the mixture was washed with methylene chloride (3×200 mL). The combined organics were washed with water (200 mL), dilute HCl (1% aq., 200 mL), sodium carbonate (satd, 200 mL) and brine (200 mL). The organics were dried with magnesium sulfate, filtered and rotoevaped to give a white solid. The solid was crystallized with hot ethanol to give white crystals (9.94g, 25.5 mmol, 76% overall yield from 2-methoxyestradiol).
[0084] Selected spectra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com