Hydro cyano and cyano fullerene derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Acid Source / Proton-Source Agent (Hydrogen cyano fullerenes)
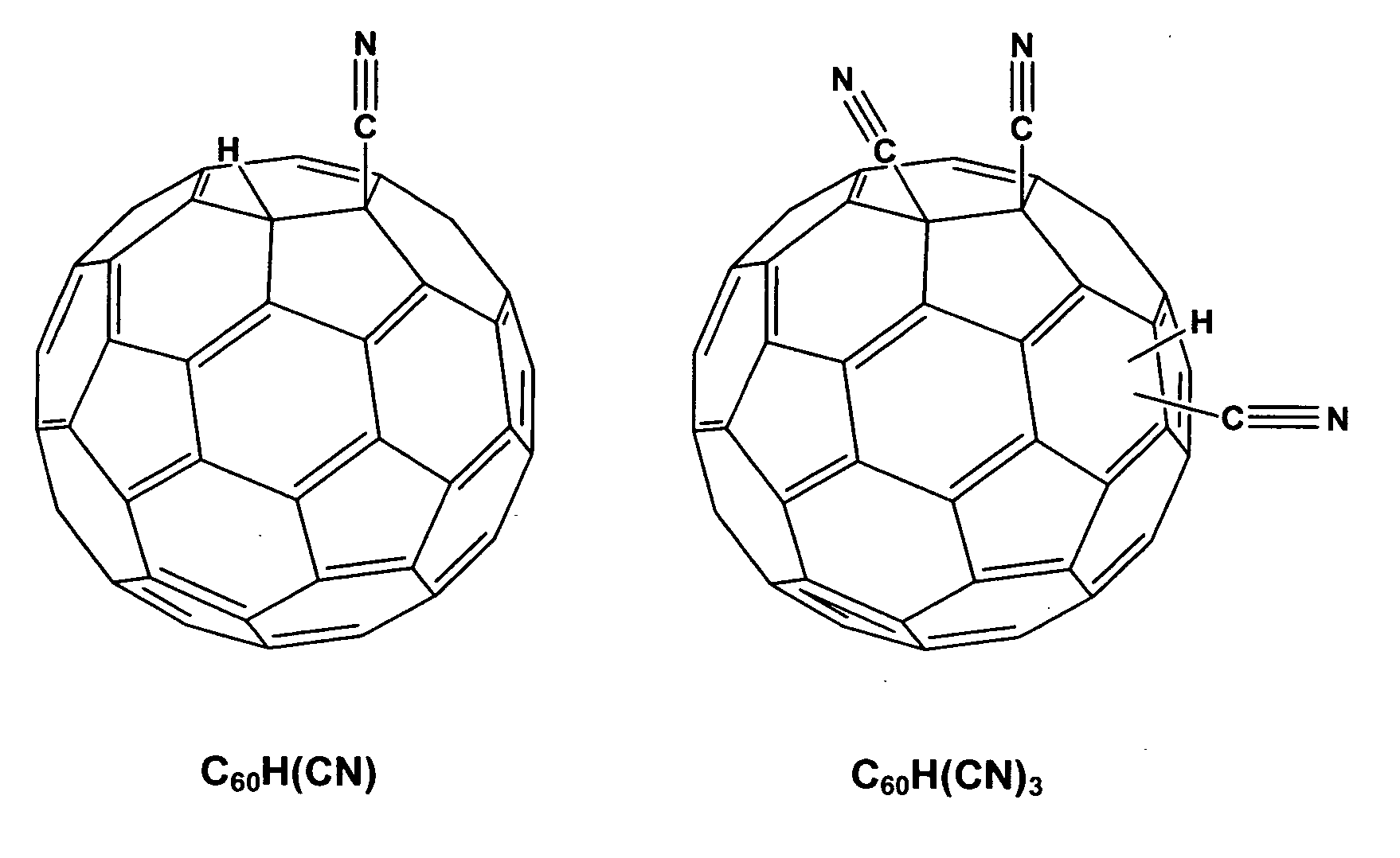
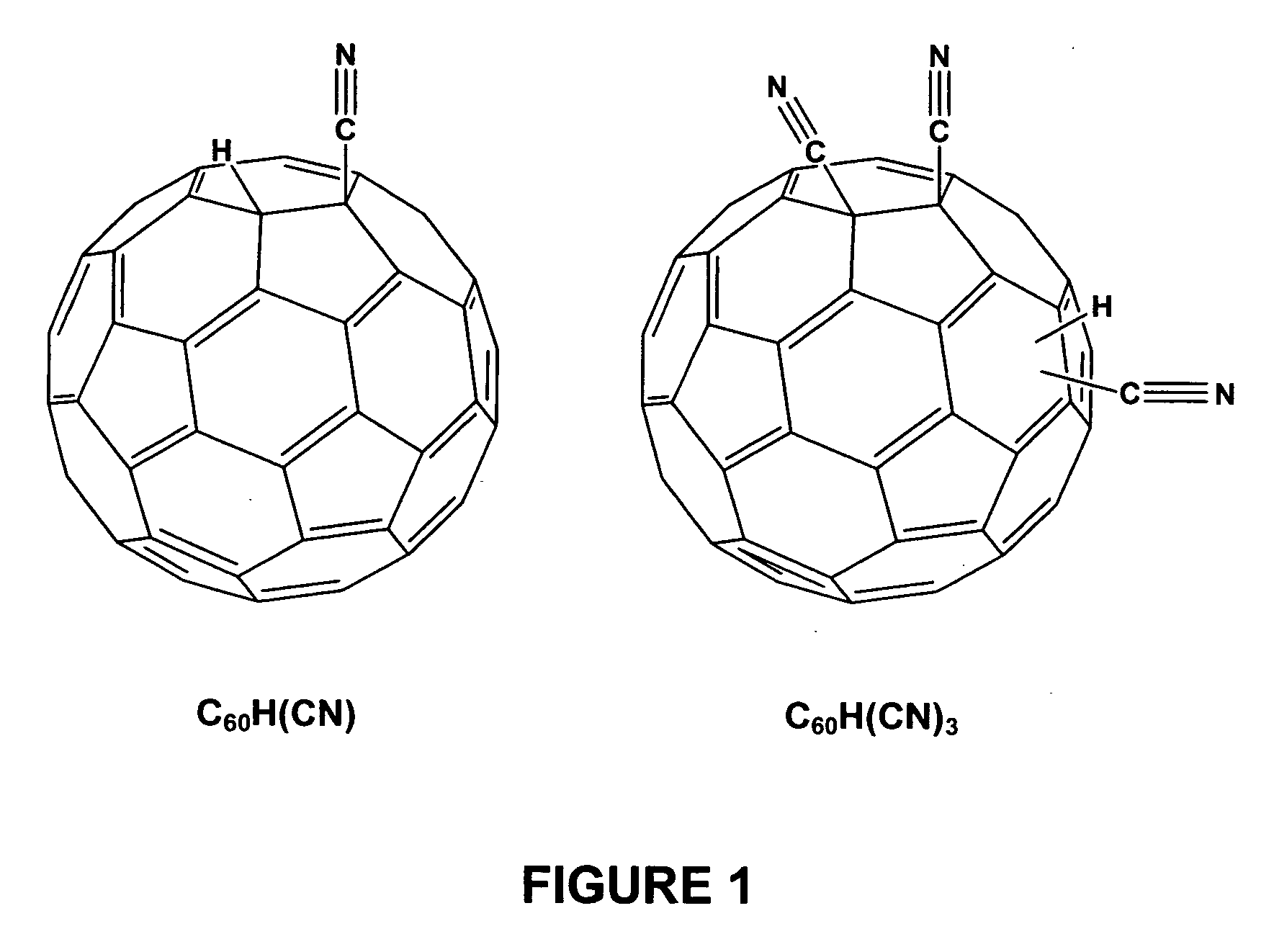
[0053] Again, C60H(CN) and C60(CN)2 were synthesized according the literature (Keshavarz, M., Knight, Srdanov, G, and Wudl F., JACS 1995, 11371).
[0054] In particular, for the preparation of C60H(CN)3 a degassed solution of NaCN (20 mg, 1.2 eq.) in DMF (20 mL) was added to a degassed solution of C60(CN)2 (260 mg, 0.34 mmol.) in ODCB (30 mL) via canula at room temperature. After being stirred 3 minutes, the resultant deep green solution was treated with perchloric acid (0.25 mL). After 30 minutes, the brown mixture was concentrated and the solid obtained was chromatographed on silica gel (CS2 / Toluene (1:3)), C60H(CN)3 was dissolved in ODCB and crystallized by adding ethyl ether or methanol (51% yield). It is noted that during the synthesis of C60H(CN)3, that the acidity of trifluoroacetic acid (pKa: 0.52) is not strong enough to protonate the C60(CN)3− and a stronger acid like perchloric acid (pKa: −1.6) w...
example 2
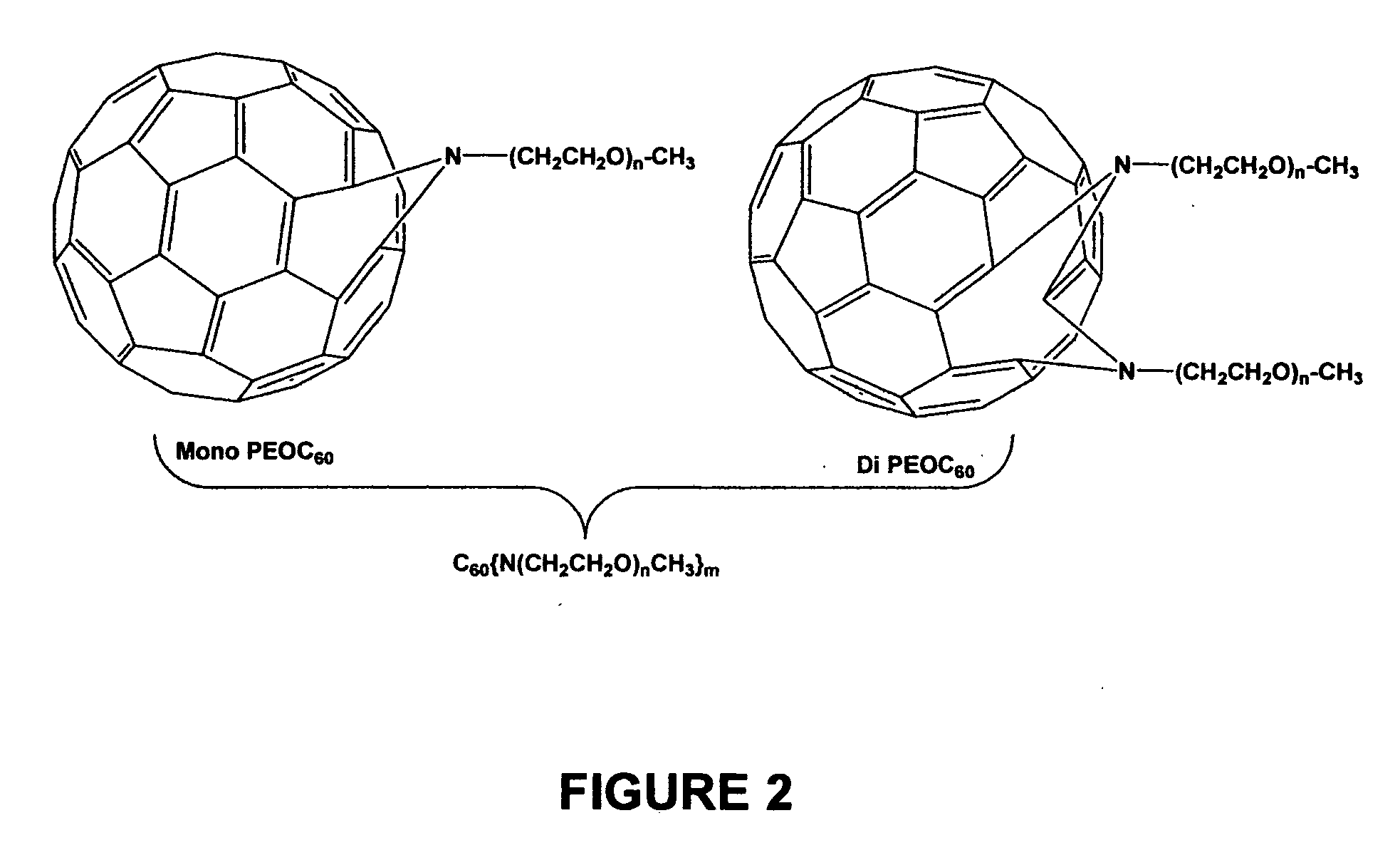
Preparation of the Mixing Agent (Poly(ethylene oxide) Attached Fullerenes)
[0060] Poly(ethylene oxide) monomethyl ethers (for example, where n˜3, 8, 12, 17, and 45) were functionalized with benzyl bromide in three steps as shown immediately below in Scheme 1:
[0061] As seen in FIG. 5, in the ATRA step, the fullerene was first dissolved in o-dichlorobenzene (ODCB) in a pressure vessel, then 8 equivalents of PEO-benzylbromide (one equivalent yields a mono-PEO final product and the like) and 2,2′-bipyridine were added and the solution was degassed for 10 minutes. After 8 equivalents of Cu(I)Br was added, the vessel was sealed and heated to 110° C. for 24 h until a green precipitate formed. Air was bubbled through the reaction mixture to precipitate un-reacted copper (I) complex. Upon filtration, the solution was concentrated and precipitated into 200 ml of ether. The product, with “n” final PEO chains and “y” bromines, was collected by filtration as a brown oil or solid (final yield w...
example 3
Membrane / Film Preparation
[0069] 1. Appropriate amounts of the C60(CN)3H (it is noted that any hydrogen cyano fullerene may be used for the exemplary C60(CN)3H proton-source agent) and, if desired, PEOmC60 (mixing agent) were weighed and added to ˜5 g of Chlorobenzene.
[0070] 2. Required amount of any desired PEO (host polymer) was added to ˜5 g of chlorobenzene in a separate container.
[0071] 3. These mixtures were sonicated (˜10 mins).
[0072] 4. They were then stirred in an 85° C. oil bath for 1˜2 hours.
[0073] 5. After confirming complete dissolution, they were mixed together and stirred for about 1 hour at 85° C. in an oil bath. (PEO tends to gel if the mixing in the earlier stages is not proper.)
[0074] 6. The resultant homogeneous solution was poured into a TEFLON dish and dried in a 120° C. oven for 2˜3 hours to get a composite film.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com