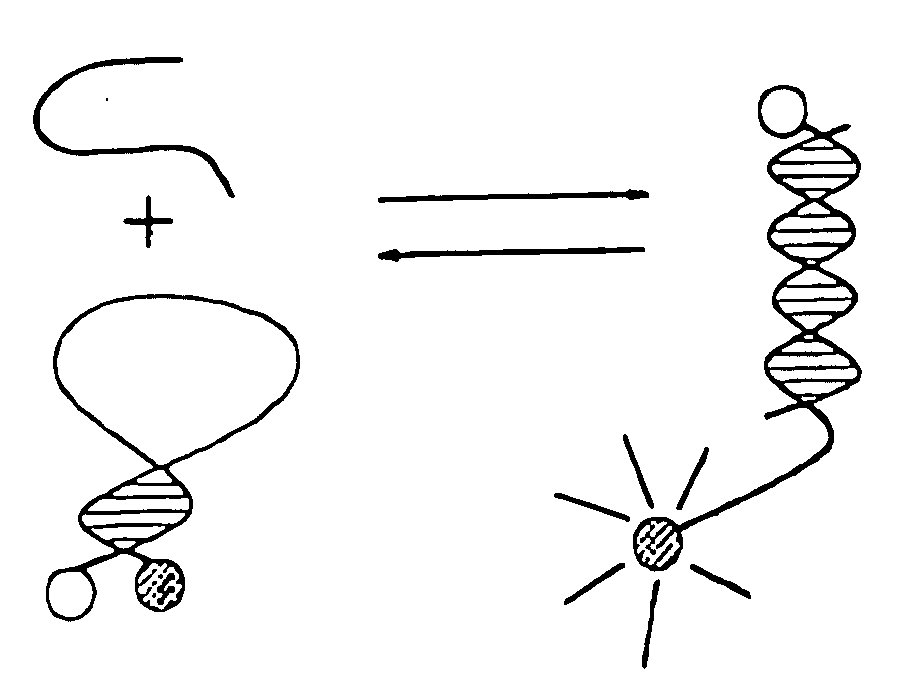
Method using a nonlinear optical technique for detection of interactions involving a conformational change
a nonlinear optical and conformational technology, applied in the field of nonlinear optical techniques for detection of interactions involving conformational changes, can solve the problems of high cost to users, difficult to assign small measured changes in a particular polarization direction to conformational changes, and difficult to separate small changes in probe orientation from large fluorescent background that may be present in many biological samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 6.1
[0480] A Molecular Beacon analogue (MB analogue) oligonucleotide, coupled to a nonlinear-active dye, and purified, is purchased from a commercial source such as Midland Certified Reagent Company (Midland, Tex.). The nonlinear-active oxazole dye used is oxazole (SE) 1-(3-(succinimidyloxycarbonyl) benzyl)-4-(5-(4-methoxyphenyl) oxazol-2-yl)pyridinium bromide (PyMPO, SE: Molecular Probes Corp.) attached via an amine group at the 3′ end.
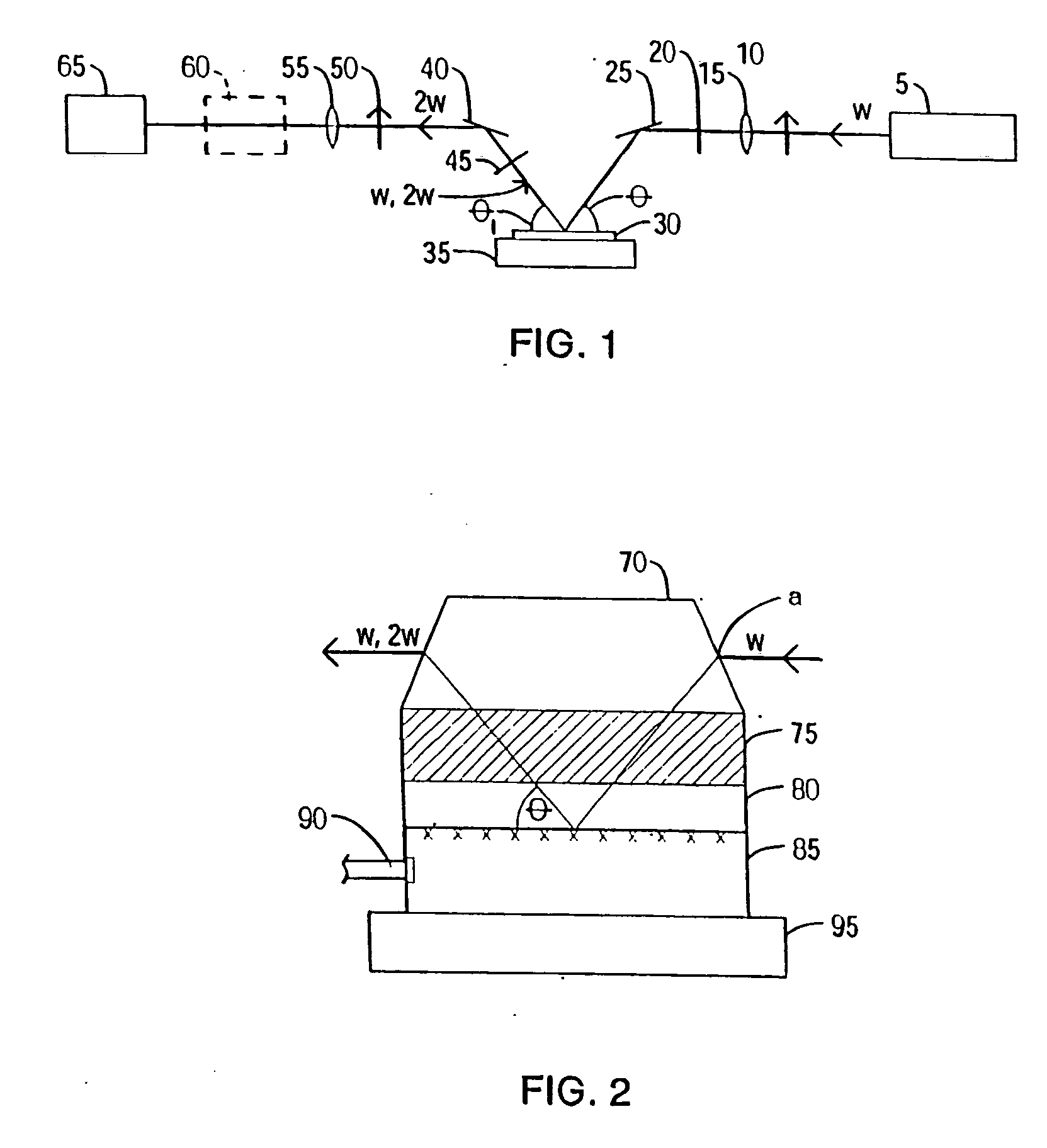
[0481] The oligonucleotide is placed into the sample well of an EFISH cell. There are a variety of EFISH cells available in the art. The sample cell described in the publication by C. G. Bethea (‘Experimental technique of dc induced SHG in liquids: measurements of the nonlinearity of CH2I2”, Applied Optics 1975, 14, 1447) is used. The direction of the applied electric field is parallel to the electric field of the laser beam. A commercial femtosecond mode-locked system (Mira 900 and Verdi 5W) is used as the fundamental source. The fundamental is directe...
example 6.2
[0484] The β2 adrenergic receptor, a GPCR protein, is purified and detergent-solubilized according to well known procedures (e.g., Ghanouni et al., 98(11): 5997 PNAS). The protein is labeled at an endogenous cysteine (Cys-265) with 1-(2,3-epoxypropyl)-4-(5-(4-methoxyphenyl)oxazol-2-yl)pyridinium trifluoromethanesulfonate (PyMPO epoxide, Molecular Probes), a nonlinear-active dye, at 1:1 stoichiometry following standard procedures and using the work of Ghanouni et al., 98(11): 5997 PNAS, as a guide. The nonlinear-active dye is attached to a part of the protein that undergoes a conformational change when the protein is activated. After separation of the non-covalently bound dye, the receptor is placed in a medium situated between two electrodes and through which passes a fundamental beam (e.g., output of ˜1 W avg. Power, ˜150 fs pulses from a Ti:Sapphire system such as the Verdi-Mira commercial system from Coherent Inc.). The apparatus and sample preparation (e.g., application of elect...
example 6.3
[0492] Oligodeoxyribonucleotides with suitable structures for molecular beacons are selected and synthesized according to procedures known to one of ordinary skill in the art with a primary amine at the 3′ end and a disulfide group at the 5′ end and a biotin group that replaces a dT. The following MB analogue can be used, for example: 5′-CCT AGC TCT AAA TCG CTA TGG TCG CGC(Biotin dT)AG G-3′ (SEQ ID NO: 6). The amine-reactive nonlinear-active oxazole dye: oxazole (SE) 1-(3-(succinimidyloxycarbonyl) benzyl)-4-(5-(4-methoxyphenyl) oxazol-2-yl)pyridinium bromide (PyMPO, SE: Molecular Probes Corp.) is conjugated to the primary amine. In this coupling reaction, a 100 μl solution containing 100 μM oligonucleotide dissolved in 0.1 M sodium bicarbonate is reacted with 0.1 mg of the succinimidyl ester of the dye dissolved in 100 μl of dimethyl sulfoxide. The reaction mixture is stirred at room temperature for 2 hours. The reaction product is purified with a Sephadex column (NAP-5; Amersham Ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| focal length | aaaaa | aaaaa |
| focal length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com