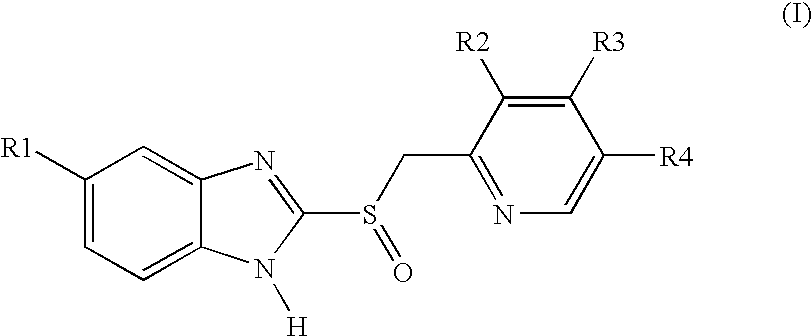
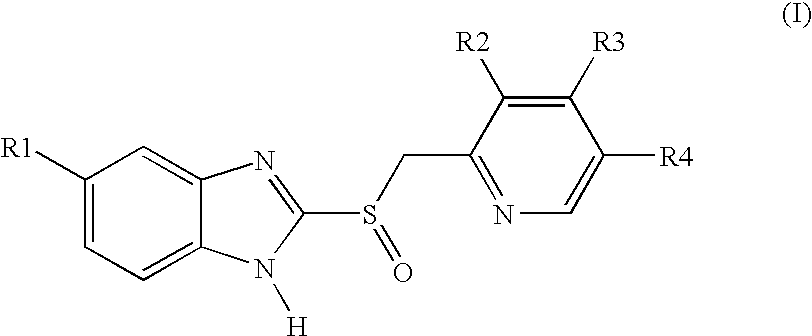
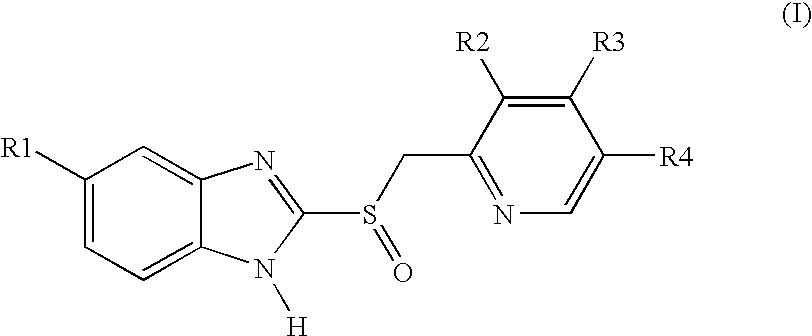
Pellet formulations of acid-labile benzimidazonle compounds
a technology of benzimidazole and pellet, which is applied in the field of pellet formulations of acid-labile benzimidazole compounds, can solve the problems of increasing the ph of stability, the need to protect the active ingredient of the pharmaceutical active ingredient from the enteric coating, and poor stability of the benzimidazole compound over tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Industrial Preparation Process of Pellet Formulations of Omeprazole and Lansoprazole
[0025] Batches of 600 kg of a pellet formulation of the benzimidazole compound (omeprazole or lansoprazole) were prepared in a standard film-coating machine according to the following steps: [0026] Step 1: Inert granules (302.40 kg, 0.7-0.9 mm diameter) of sucrose (80%) and starch (20%) were introduced In the machine and warmed to 30-35° C. [0027] Step 2: An aqueous solution of sodium carboxymethyl starch (Explotab®; 11.34 kg), polyvinylpyrrolidone (29.925 kg) and sodium lauryl sulfate (18.27 kg) was prepared by addition of the ingredients to enough water. [0028] Step 3: In a separate vessel, an aqueous solution of potassium oleate and oleic acid was prepared by dissolving 4.2525 kg of oleic acid and 0.214 kg of potassium hydroxide in water. [0029] Step 4: The solution of Step 3 was added to the solution of Step 2 until an homogeneous solution was obtained. [0030] Step 5: The required amount of benz...
example 2
Comparative Dissolution Tests
[0047] Pellet formulations with the same amount of omeprazole or lansoprazole prepared according to the process of Example 1, and commercially available pellet formulations with the same amounts of the same pharmaceutical active ingredient, were submitted to respective comparative dissolution tests in aqueous solutions of pH 6.8, in the same apparatus, stirring at the same speed (100 rpm), at the same temperature (37° C.), and for the same times.
[0048] A lansoprazole pellet formulation prepared according to Example 1 showed similar dissolution profiles than commercial Opiren® (lansoprazole from Almirall Prodesfarma, under license of Takeda). Thus, after 15 min, percentages of lansoprazole dissolved were: Opiren®, 58.4%; present invention, 69.3%. And after 30 min, percentages were: 71.3% and 86.5%, respectively. All pellets had been obtained by coating of inert nuclei, and those under license from Takeda presumably had been obtained with the process des...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Molar ratio | aaaaa | aaaaa |
| Stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com