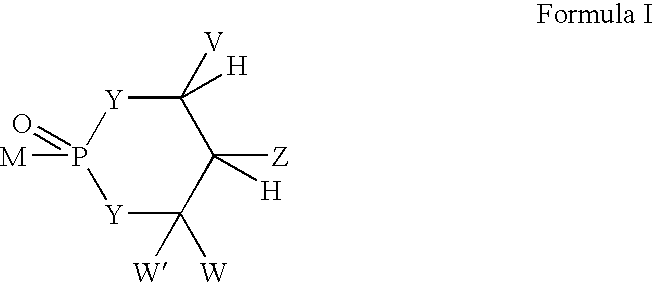
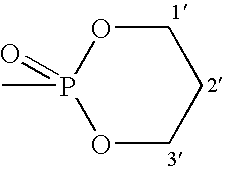
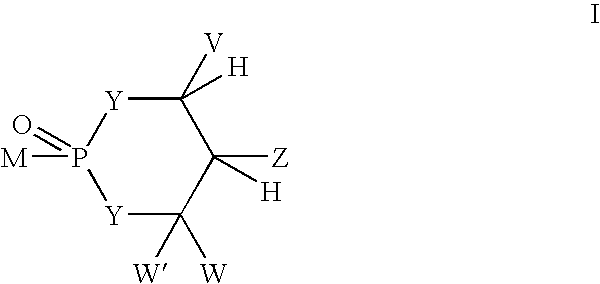
Novel phosphorus-containing prodrugs
a phosphorus-containing, prodrug technology, applied in the field of new phosphorus-containing prodrugs, can solve the problems of poor oral bioavailability, poor cell penetration and limited tissue distribution, poor drug effect, etc., to reduce the production of enzymes, reduce the activity of phosphorylating enzymes, and improve the effect of drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for Phosphoramidate Prodrugs of Phosphonic Acid Containing Drugs
[0674] A suspension of 5-Isobutyl-2-methyl-4-(2-(5-phosphono)furanyl)thiazole (200 mg, 0.6 mmol) in 2.5 mL of thionyl chloride was heated at reflux temperature for 4 h. The reaction mixture was cooled and evaporated to dryness followed by azeotroping with toluene. To the solution of the resulting residue in 4 ml of methylene chloride was added a solution of amino alcohol (82 mg, 0.54 mmol) and pyridine (0.5 ml, 6 mmol) in 1 mL of methylene chloride.
[0675] After stirring at 25° C. for 4 h the reaction was evaporated and coevaporated with toluene. The crude product was chromatographed by eluting with 10% methanol-dichloromethane to give 52 mg (20.8%) of a less polar isomer and 48 mg (19.2%) of a more polar isomer.
[0676] The following compounds were prepared in this manner:
[0677] 1.1: 5-Isobutyl-2-methyl-4-{2-[5-(1-phenyl-1,3-propyl)phosphorimido]furanyl}thiazole, less polar isomer. Rf=0.76 in 10% MeO...
example 2
Step A:
[0688] A solution of 3-amino-3-phenyl-1-propanol (1 g, 6.6 mmol) and triethylamine (3 ml, 21.8 mmol) in tetrahydrofuran (60 ml) was added dropwise over 20 minutes into a solution of 4-nitrophenyl phosphorodichloridate in tetrahydrofuran at 0° C. A white precipitate quickly forms. The yellow heterogeneous mixture was stirred at 0° C. for 1 hour then warmed slowly to rt. After stirring at rt for 60 hours, the precipitate was dissolved with water (40 ml). The clear yellow solution was concentrated to ca 40 ml. The resulting mixture was diluted with a saturated aqueous solution of sodium bicarbonate and extracted twice with ethyl acetate. The combined organic extracts were washed with a saturated aqueous solution of sodium bicarbonate and brine. The aqueous washes were back extracted and the organic extracts combined. The combined organic extracts were dried over sodium sulfate, concentrated and purified by column chromatography (silica gel, hexanes / ethyl acetate) to give fast ...
example 3
Step A:
[0692] Same as step A of example 2.
Step B:
[0693] Lithium hydride (15 mg, 1.52 mmol) was added to a solution of 9-β-D-arabinofuranosyl-adenine (100 mg, 0.37 mmol) and fast eluting (4-nitrophenoxy)-4-phenyl-1,3,2-oxazaphosphorinan-2-one (250 mg, 0.74 mmol) in dimethylformamide at room temperature. After 6 hours the yellow heterogeneous mixture was neutralized with acetic acid. The clear yellow solution was concentrated and the residue was purified by column chromatography (silica gel, methanol / dichloromethane). The isolated product was further purified by preparative TLC and re crystallized from ethanol to give the prodrug: 18 mg (11% yield);
[0694] The following compound is prepared in this manner:
[0695] 3.1 5′-O-(2-oxo-4-Phenyl-1,3,2-oxazaphosphorin-2-yl)-9-β-D-arabinofuranosyl-adenine. mp>250. Rf=0.25 (silica gel, 2 / 8 methanol / dichloromethane). Anal. cald. for C19H23N6O6P: C: 49.35; H: 5.01; N: 18.17. Found: C: 49.22; H: 4.89; N: 18.03.
[0696] The following compounds ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com