Method for Regulating Production of Hemoglobin Beta Chains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0123]Materials and Methods
[0124]Cell lines. K562 (human erythroleukemia) cells were grown in suspension in RPMI 1640 medium with 10% or 15% fetal bovine serum (FBS) and antibiotics as described (Berg et al., (1989) Nucleic Acids Res 17, 8833-52) and harvested at a density of 10.sup.6 cells / ml for making nuclear extracts. CV-1 (African green monkey kidney epithelial) cells (adherent cells used for transfections / transient gene expression assays) were grown in DMEM with L-glutamine, 10% FBS and antibiotics (Miller & Bieker (1993) Mol Cell Biol 13, 2776-86).
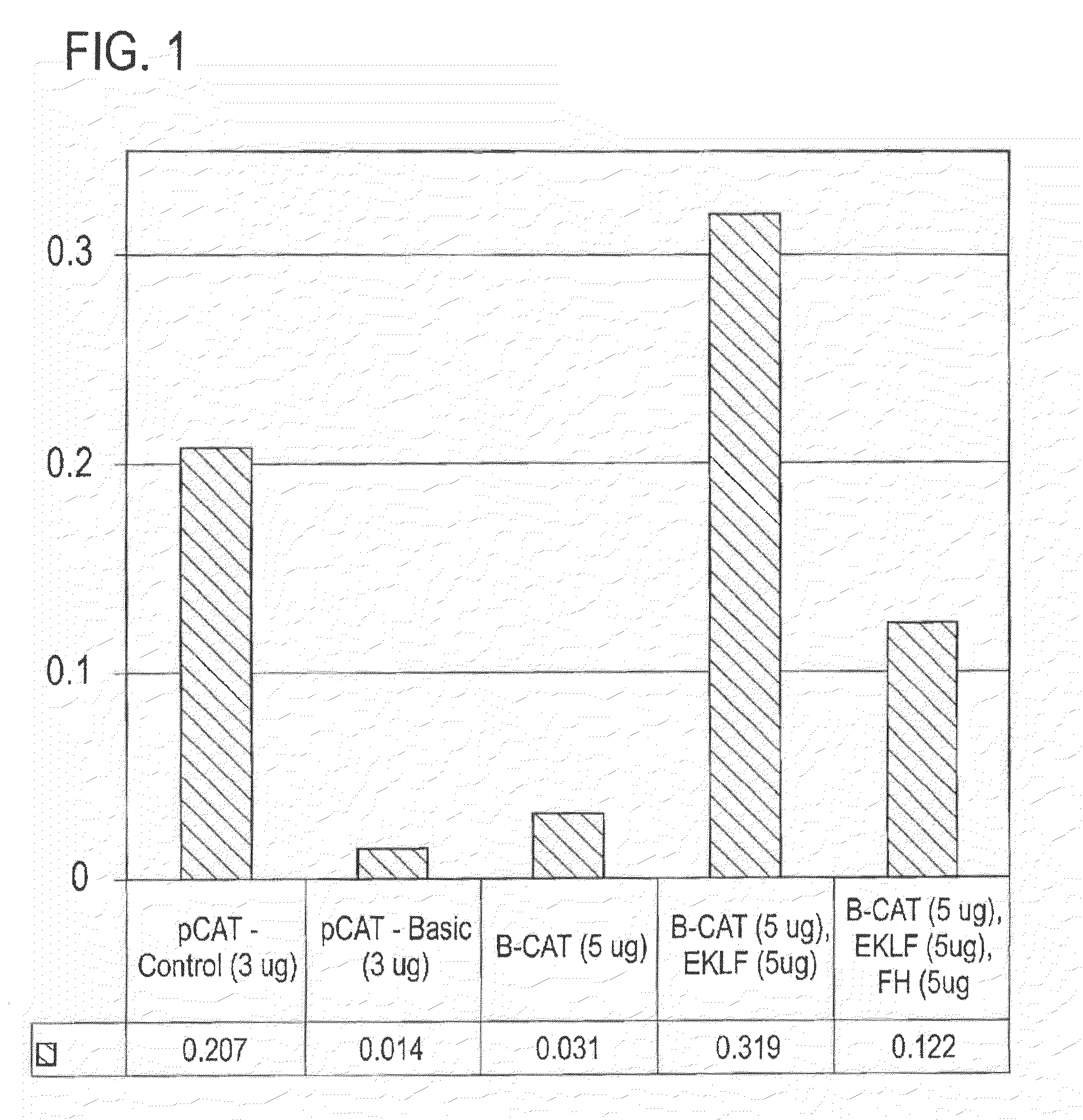
[0125]Clones, transfections, and gene expression assays. The upstream region (−610 / +20) of the human β-globin gene, previously cloned in pSV2CAT (Berg et al., (1989) Nucleic Acids Res 17, 8833-52), was subcloned through pGEM and pSELECT (now called pALTER) and recloned into pCAT-basic (all vectors from Promega). Mutants of the −153 / −148 site of the β-globin promoter were generated by transcription from mutant oligonucleotides corres...
example 2
[0147]Materials and Methods
[0148]Materials: Calf intestine alkaline phosphatase, T4 polynucleotide kinase, and Sau 96 I were obtained from Promega / Fisher. 32P-γ-ATP was from Dupont / NEN. Polyclonal (rabbit) antiserum to human spleen ferritin was obtained from Sigma Chemical Company. All other reagents were molecular biology grade.
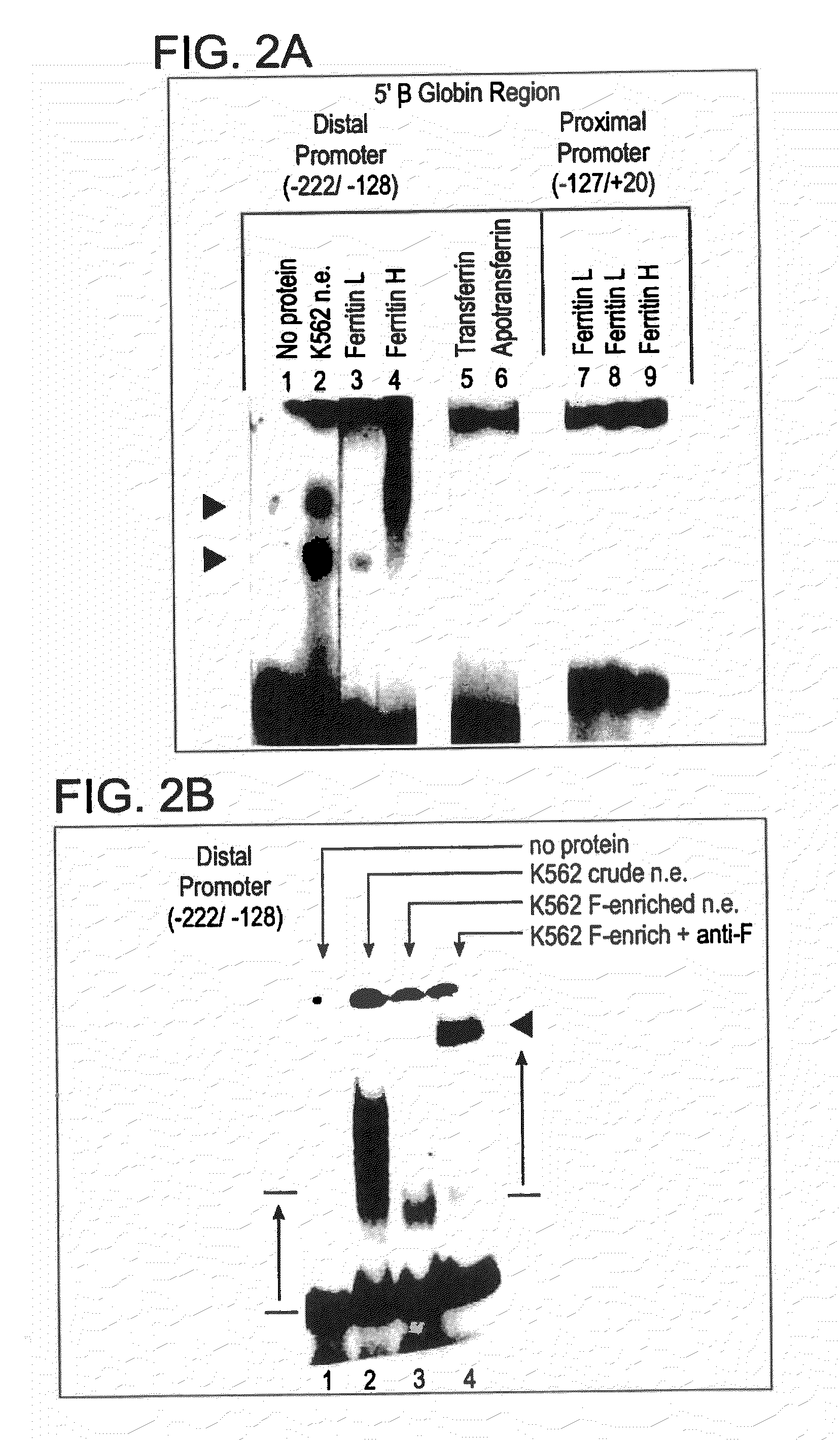
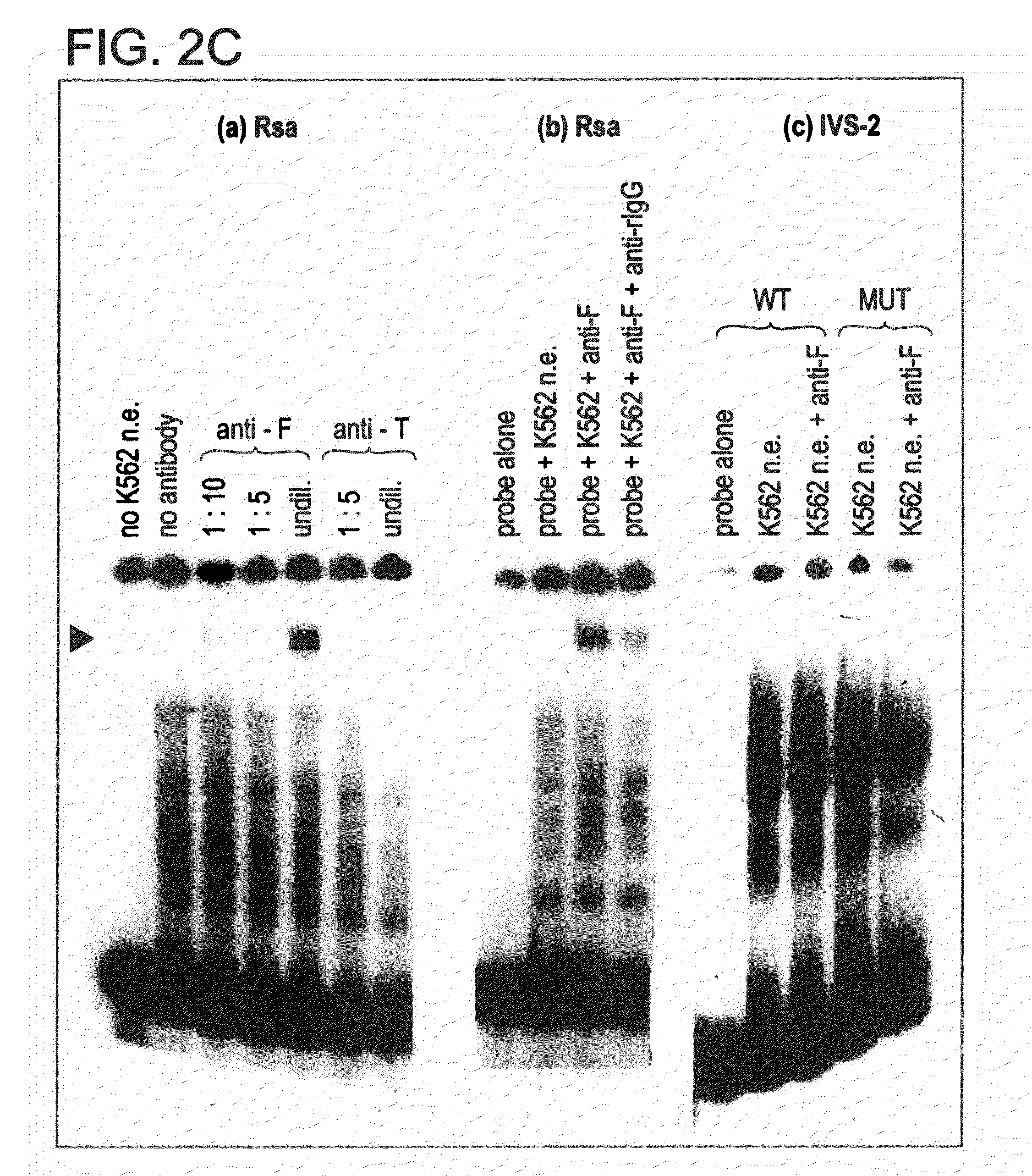
[0149]Restriction fragments and oligonucleotides: The 5′ region of the human β-globin gene (from −610 to +20), previously cloned in pSVOCAT, was cut from the purified plasmid by digestions with Hind III and Bam HI. The 630 bp fragment was phenol / chloroform treated, dephosphorylated, and end-labelled with 32P. Synthetic oligonucleotides corresponding to the core / BP-1 binding site of NCR1 (−584 / −527), the more distal of the two 5′-β-globin silencers, and −164 / −128 region of the promoter were purified and annealed, and the double-stranded oligos were end-labeled as above and / or used as unlabeled competitors in gel mobility shift assays.
[0150]Preparation of nucl...
example 3
Human Ferritin-H Localizes to the Nucleus of Primate Cells
[0167]Materials and Methods
[0168]Cell lines. CV-1 (African green monkey kidney epithelial) cells (adherent cells used for transfections / transient gene expression assays) were grown in DMEM with L-glutamine, 10% FBS and antibiotics (Miller, I. J. & Bieker, J. J. (1993) Mol Cell Biol 13, 2776-86).
[0169]Clones, transfections, and gene expression assays. Ferritin-H from the pcEXV-1 plasmid was PCR-amplified then cloned in the vector pCR4-TOPO. Ferritin-H was then digested using newly created restriction sites (Bse AI and BamHI), and cloned into the pEGFP-C1 vector to create a GFP-FtH fusion protein for expression in mammalian cells. Transfections of CV-1 cells were carried out with DMRIE-C transfection reagent, fluorescent protein plasmid pEGFP-C1 (Clontech), fluorescence microscopy and quantitative fluorescence of cell lysates with a microtiter plate reader.
[0170]Microscopy. Cells transfected with EGFP (enhanced green fluorescen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com