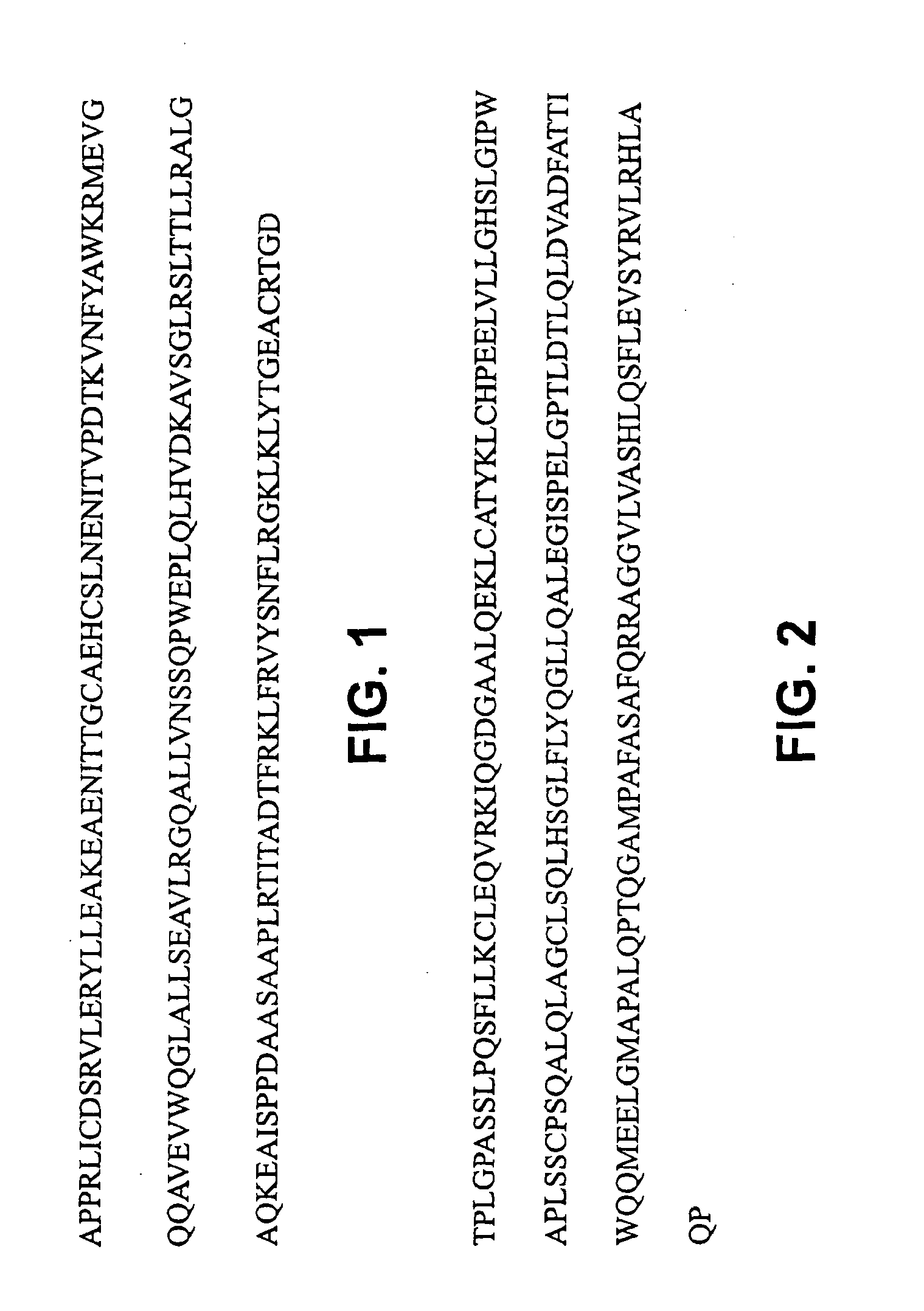
Acryloyloxyethylphosphorylcholine Containing Polymer Conjugates and Their Preparation
a polymer and phosphorylcholine technology, applied in the field of polymer reagents and conjugates, can solve the problems of short biological half-life, unsatisfactory forms, painful and/or inconvenient, etc., and achieve the effect of reducing steric hindrance, preventing or reducing the efficiency of bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-Ethylacrylic Acid From Diethyl Malonate
[0381]Ethylacrylic acid was prepared from diethyl malonate by preparation of 2-carbethoxybutyric acid that was subsequently converted into 2-ethylacrylic acid (EAA).
2-Carboethoxybutyric Acid
[0382]Diethyl ethylmalonate was stirred overnight with 95% ethanol in the presence of 1M KOH. The white precipitate that forms was filtered out of solution, and the filtrate was concentrated under reduced pressure (e.g., on a rotary evaporator). The yellowish oil obtained upon concentration of the filtrate was added to the precipitate and the mixture was dissolved in a minimum amount of water. After acidification with dilute aqueous HCl to a pH of 2, an oil separates from the solution. The oil was taken up into diethyl ether and the aqueous layer was extracted three times with further with diethyl ether. The ether extracts 0.92 were combined, dried over magnesium sulfate and filtered. A quantitative yield of 2-carboethoxybutyric acid was obt...
example 2
Preparation of 2-methacryloyloxyethyl phosphorylcholine (HEMA-PC or MPC)
[0384]
[0385]To a solution of 2-hydroxyethyl methacrylate (HEMA) (4.4 g; 40 mmol) in dry THF (40 mL) was added dry triethylamine (3.4 g, 40 mmol). This mixture was stirred at room temperature for 3 minutes, then cooled to −20° C. To the cooled solution was added COP (4.5 g, 40 mmol) as a solution in THF (20 mL) over a period of 1 hour. The temperature of the reaction mixture was maintained around −20° C. The white precipitate was filtered, and the remaining solution concentrated under reduced pressure to yield 6.7 g (71%) of 2-(2-oxo-1, 3, 2, dioxaphospholoyloxy)ethylmethacrylate (OPEMA) as pale yellow liquid. OPEMA (4.0 g, 16 mmol) was stirred in dry acetonitrile (30 mL) in a reaction bottle. The bottle was cooled to −20° C., and anhydrous trimethylamine (4 mL) was added rapidly to the solution. The bottle was closed quickly, and the mixture heated to 60° C. for 24 hours, then kept at −10° C. for 12 hours. MPC w...
example 3
Preparation of 2-Bromo-2-methyl-propionic acid pyrrolidin-1-yl ester
[0386]
[0387]A solution of N-hydroxysuccinimide (NHS) (3.0 g, 26 mmol) in dry methylene chloride (300 mL) was stirred under an inert (argon or nitrogen) atmosphere. Triethylamine (7.3 mL, 52 mmol) was added, the mixture was cooled to 0° C., and 2-bromo-2-methylpropionyl bromide (3.9 mL, 31 mmol) was added dropwise over a period of 15 minutes. The mixture was stirred for 1 hour at 0° C., then warmed to room temperature and stirred overnight. Most of the solvent was removed by evaporation, and the remaining mixture was diluted with cold water (400 mL), and extracted with diethyl ether (3×30 mL), then washed with aqueous potassium carbonate (3×30 mL) and 1M HCl(aq) (3×30 mL). The organic layer was dried over anhydrous magnesium sulfate, then filtered and concentrated to give a brown solid. Crystallization from ethyl acetate gave the desired product as a white powder (2.62 g, 39%). 1H NMR (CDCl3) 5 (ppm) 2.09 (s, 6H, CH3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com