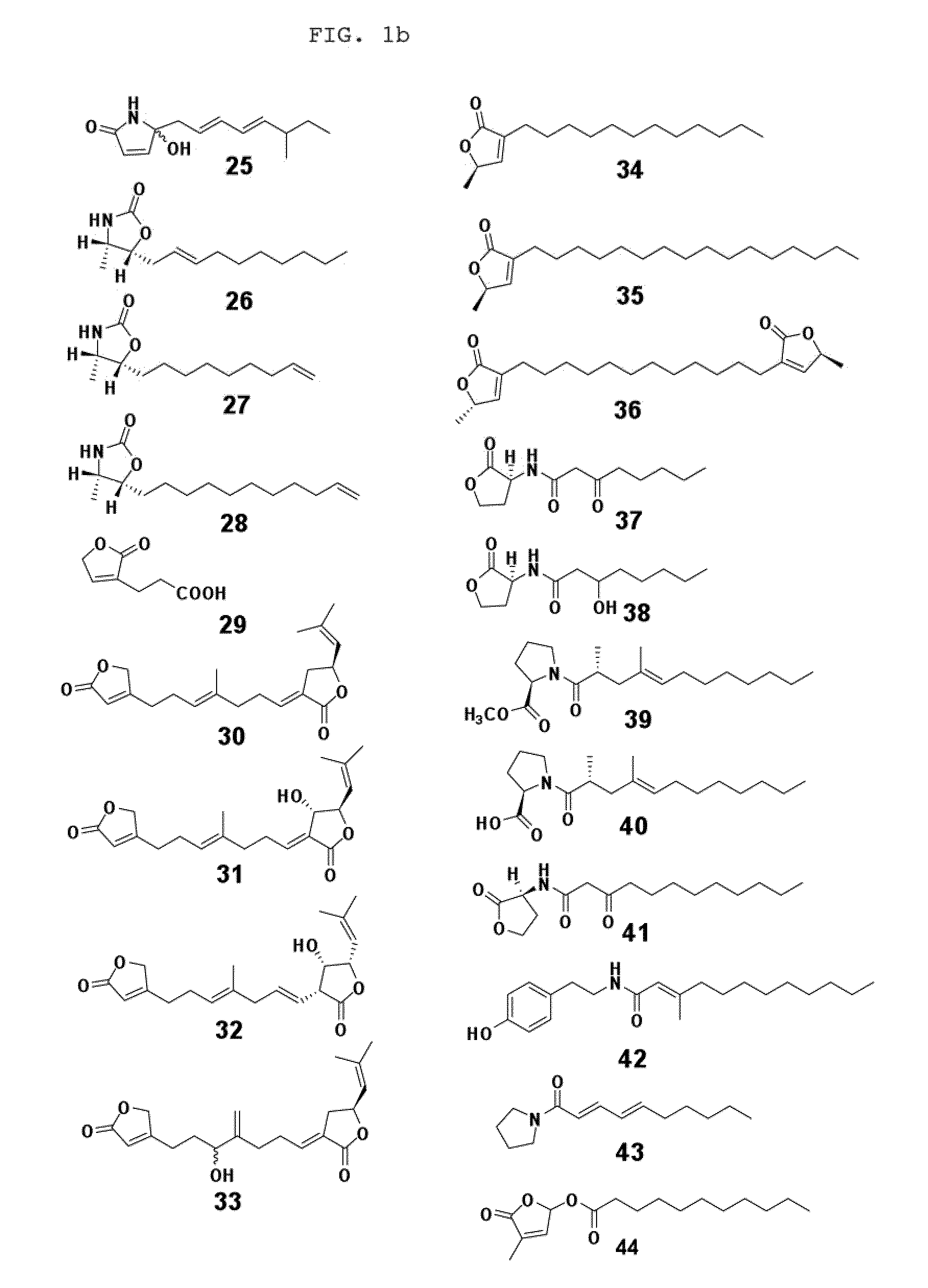
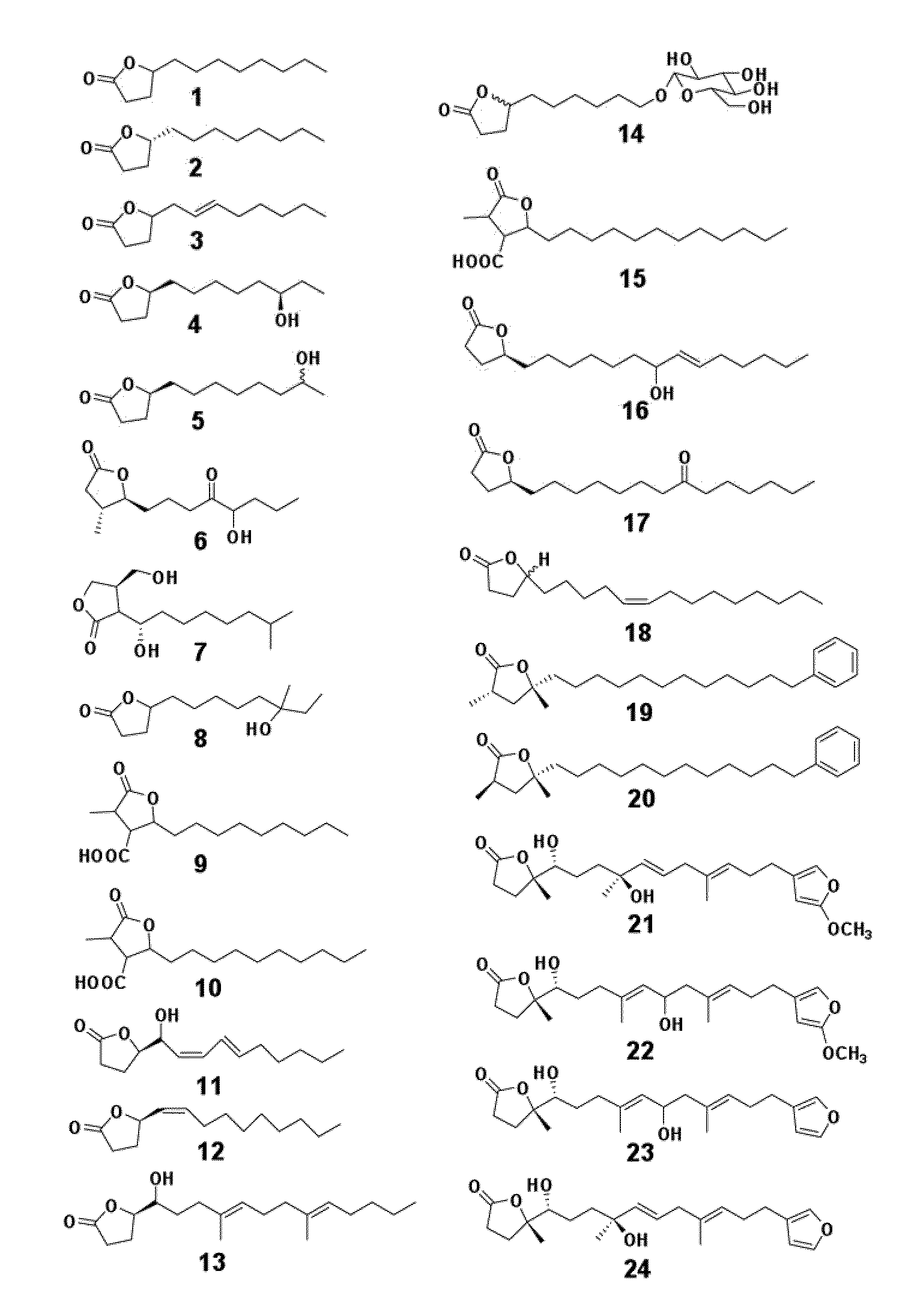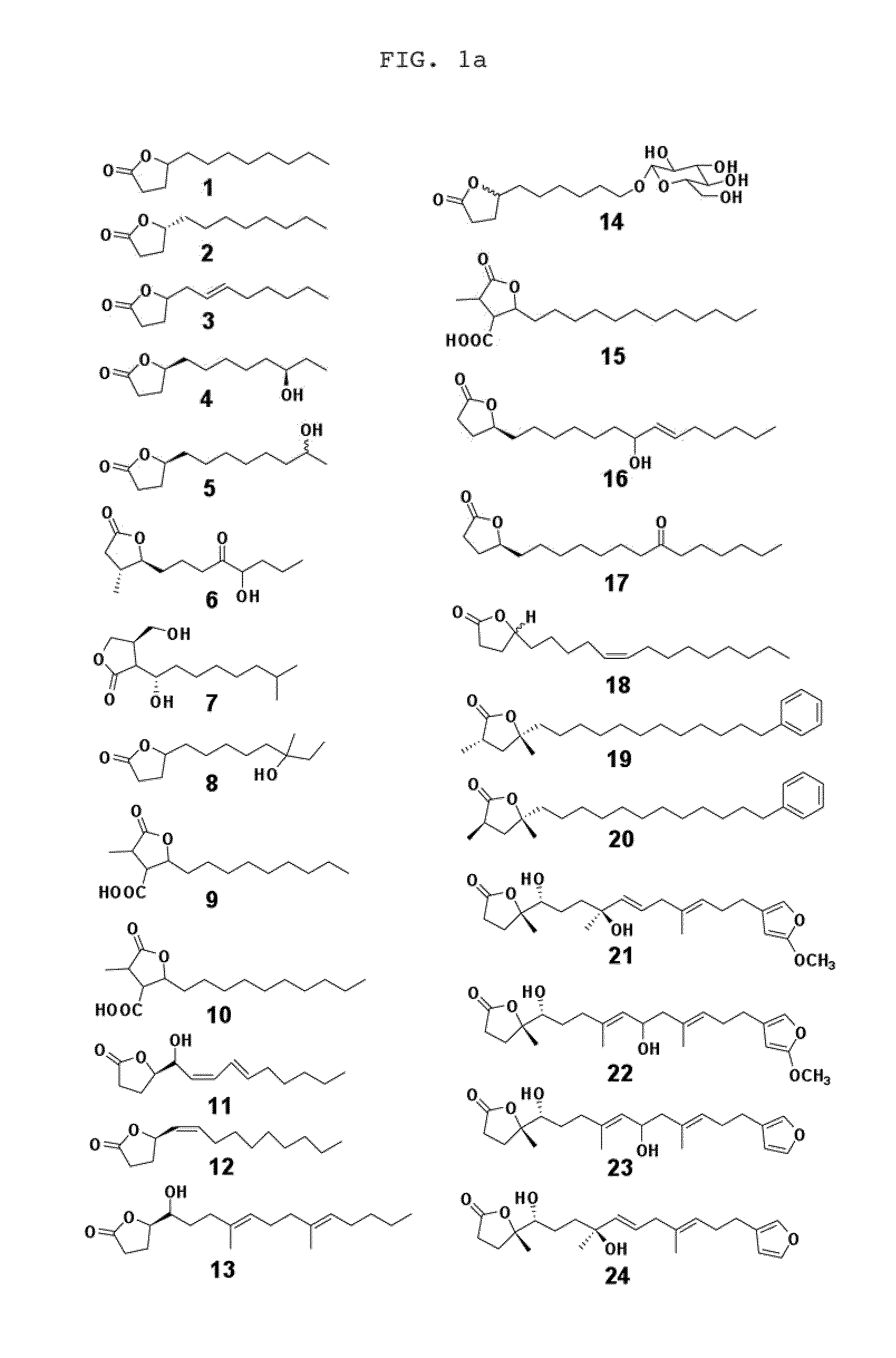Chemical and biological agents for the control of molluscs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Molluscicide Studies
Materials and Methods
[0083]1. Allow quagga mussels to acclimate in the small Petri dishes for 24 hrs.[0084]Pour the mussels into a Petri dish to determine if the mussels are alive or not. Toss out the dead and empty mussels.[0085]Count out 10 live / healthy mussels.[0086]Put 10 live / healthy mussels in each small Petri dish with hard water.[0087]Keep a separate plate of mussels. These are the “extra mussels” that will be used to replace dead or empty mussels after 24 hrs in the other experimental plates.[0088]Aeration is not needed due to the low volume of water (DO is high).
[0089]2. Day of mussel treatment:[0090]Check the mussels (Use the rubber policemen at all times when checking the mussels. Only use the tweezers to remove dead mussels).[0091]Count to make sure there are 10 live / healthy mussels per small Petri dish.[0092]Get the sample(s) ready.[0093]Dilute appropriately with hard water in a 50 ml falcon tube for each sample. Vortex to mix prior to dosing.[0094]...
example 2
Erwinia Extracts
[0122]Erwinia carotovora is grown on LB broth (per liter: 10 g tryptone, 5 g yeast extract, 10 g NaCl, pH=7.5). Inoculum is grown by streaking a TSA (tryptic soy agar) plate from a glycerol stock. Purity of the culture is confirmed through visual inspection of colony morphology. Using a sterile 10 μL loop, colonies are collected from the agar surface and resuspended in 50 ml of LB broth in a 250 ml non-baffled Erlenmeyer flask with screw cap. The liquid culture is incubated for 48-72 hours at 200 rpm and 25° C.
[0123]After 72 hr, the whole broth is extracted with ethyl acetate. The organic phase is dried under vacuum. The dried extracted is made a 5.0 mg / mL solution in dimethyl sulfoxide (DMSO). Then, such solution (100 μL) is added into 45 mL hard water. The final concentration of ethyl acetate extracts is 11.1 ppm.
[0124]Data shown in Table 3 indicates that bioactive compounds against the quagga mussels are produced in Erwinia carotovora when grown in the LB media. T...
example 3
Isolation of Molluscicidal Compounds from Pseudomonas
Study A
Fractionation of Compounds
[0125]The following procedure is used for the fractionation of compounds extracted from washed cells of Pseudomonas fluorescens CL-145A:
The cell pellet derived from the 10-L fermentationP. fluorescens CL 145A (ATCC 55799) in FM2 growth medium is suspended in dilution buffer and extracted with Amberlite XAD-7 resin (Asolkar, R. N., Jensen, P. R., Kauffman, C. A., Fenical, W. 2006. Daryamides A-C, Weakly Cytotoxic Polyketides from a Marine-Derived Actinomycete of the Genus Streptomyces strain CNQ-085 J. Nat. Prod. 69:1756-1759; Williams, P. G., Miller, E. D., Asolkar, R. N., Jensen, P. R., Fenical, W. 2007. Arenicolides A-C, 26-Membered Ring Macrolides from the Marine Actinomycete Salinispora arenicola. J. Org. Chem. 72:5025-5034) by shaking the cell suspension with resin at 225 rpm for two hours at room temperature. The resin and cell mass are collected by filtration through cheesecloth and washed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com