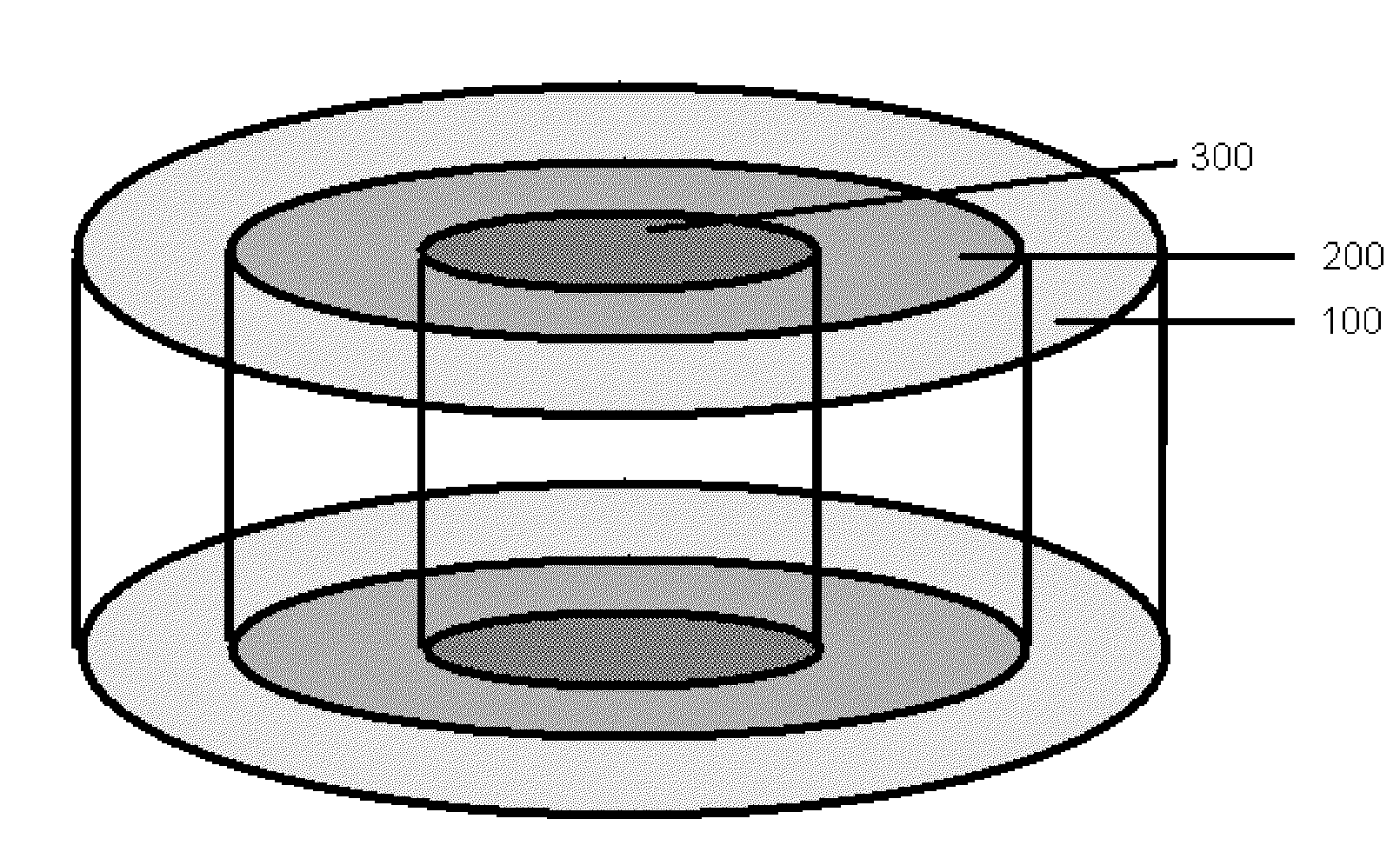
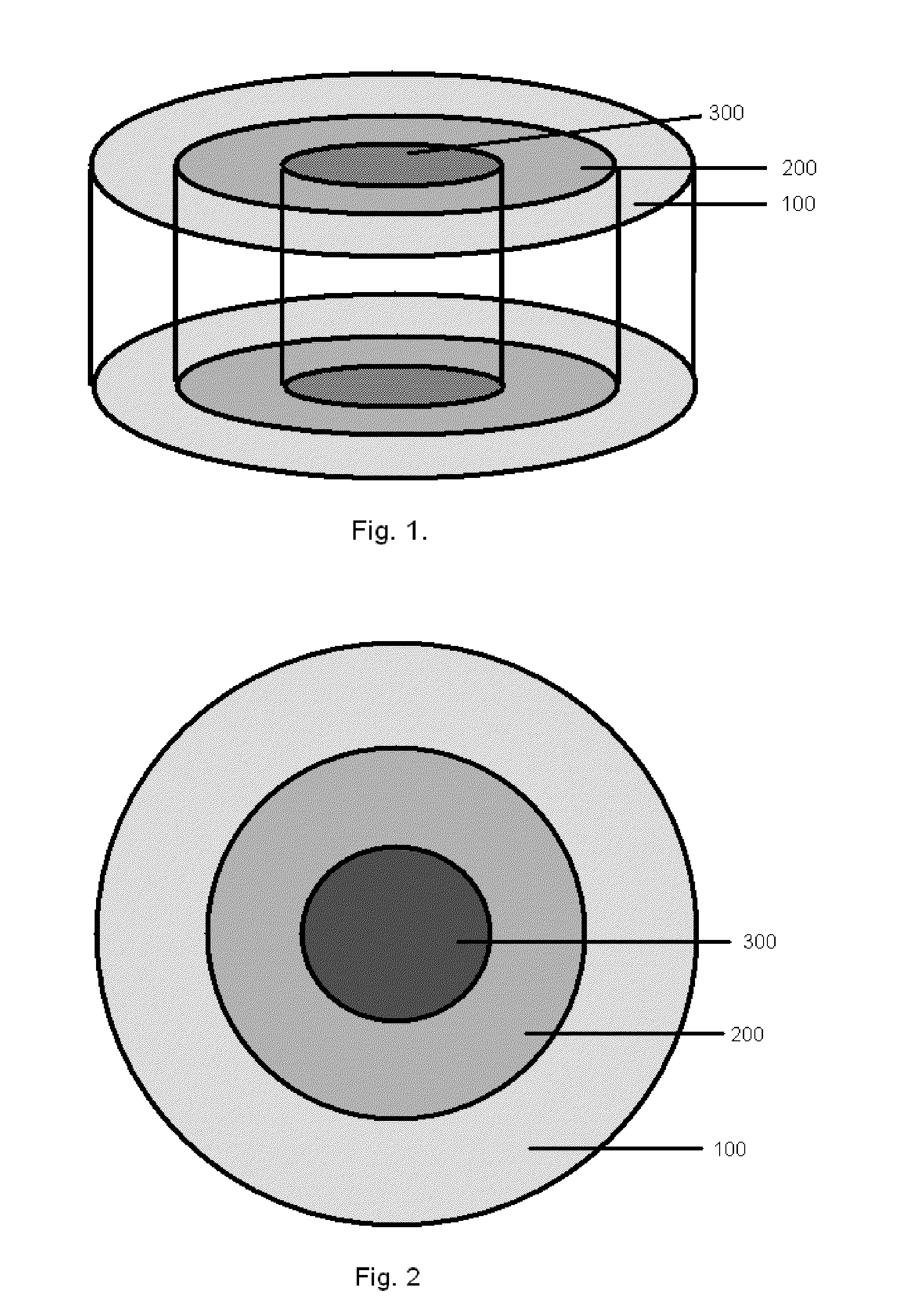
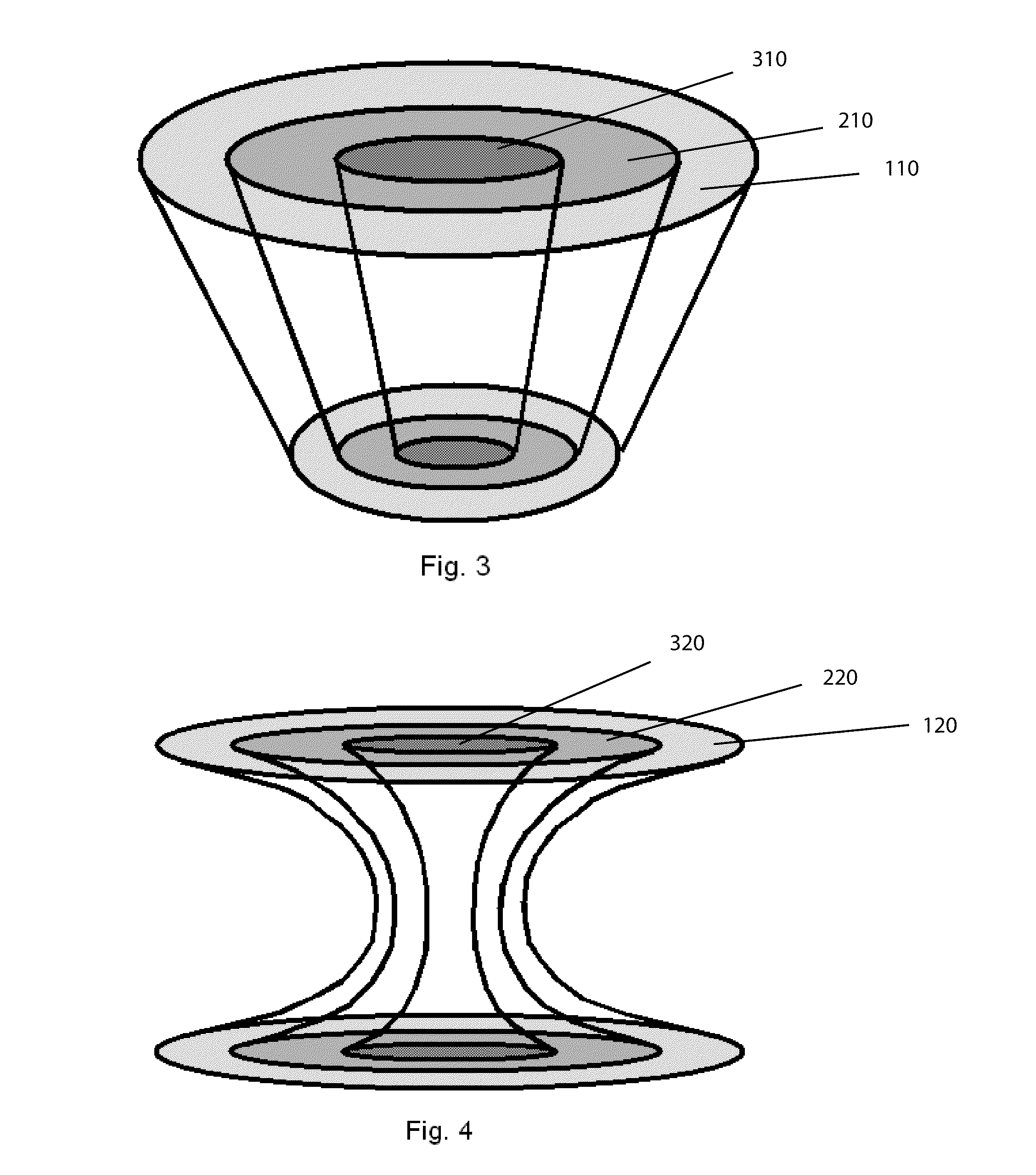
Bone is very sensitive and the sharp pain of
arthritis often comes from
irritation of bone nerve endings and since human tissue has a very
limited capacity to heal without a
blood supply, articular cartilage cannot repair itself effectively.
Without articular cartilage, stress and friction would occur to the extent that the joint would not permit motion.
As stated above, articular cartilage has only a very
limited capacity to regenerate.
If this tissue is damaged or lost by traumatic events, or by chronic and progressive degeneration, it usually leads to painful arthrosis and decreased range of joint motion.
Articular cartilage repair following injury or degeneration represents a major clinical problem, with treatment modalities being limited and
joint replacement being regarded as appropriate only for the older patient.
(Extensive use of
cortisone not only has a wide variety of harmful effects, but is also believed to harm cartilage.)
These were
safer than
Aspirin and
cortisone but had potent side effects, especially causing bleeding within the
stomach and intestinal ulcers.
While much
safer and seemingly more effective, Vioxx was found to have significant cardiac side effects and is no longer available.
However, these anti-inflammatory medications only treat the symptoms of
cartilage damage and
arthritis and do not promote repair.
Currently, hyaluronate injections are approved for the treatments of
osteoarthritis of the knee in those who have failed to respond to more conservative therapy, Once again, this procedure only treats the symptoms of
cartilage damage and
arthritis and does not promote repair.
But this is not a final solution as the degenerative process continues to wear away at the articular cartilage.
Although successful, the
window of opportunity for this procedure is often missed, as the few clinical symptoms showing the need for this treatment are not evident until the defect deepens to involve the underlying bone, thus the damage encountered upon detection is frequently too extensive for repair through ACI.
Several plugs can fill up rather larger defects and will grow to re-supply a new
joint surface.
Unfortunately, this procedure leaves defects of equal or worse proportions elsewhere and often the harvested tissue is not viable due to the traumatic harvesting procedure.
Due to the problems associated with current state of the art treatments, much work has been done to produce a synthetic off-the-shelf
scaffold to be used in place of the harvested osteochondral plug.
However, these single-phase
scaffold implants proved unsuccessful in healing of the complex multiphasic articular cartilage along with the underlying bone.
While these showed an improvement over
single phase devices, it is evident that these devices do not take into consideration how cells will be migrating into the scaffolds as well as how their presence influences the surrounding, uninvolved tissue.
Additionally, prior art scaffolds did not take into consideration the
joint fluid and how it impacts maturation and maintenance of healthy hyaline cartilage.
Although prior art synthetic scaffolds, whether
single phase, multi-phase, or of gradient construction have proven suitable for growth and maturation of cells within a
bioreactor, these prior art devices are unsuitable for direct implantation, for at least the reasons that follow.
Although some success in establishing hyaline cartilage can be seen in small defects of 5 millimeters or less, larger defects show tell tale signs of collapse or dimpling in the center of a repair plug, as the less desirable fibrocartilage, which has grown within the prior art devices, succumbs to the forces within the joint.
Additionally, prior art devices show a halo or ring of collapsed tissue around the periphery of the device do to lack of intimate contact with the uninvolved tissue that has retracted away from the defect site.
Another discovery of applicants is that prior art devices do not address the instantaneous articular cartilage tissue contraction that occurs when the surface of hyaline cartilage is
cut or torn.
Anomalous mechanical loading of these tissues often leads to
pathology.
For example, the lack of mechanical stimulation of a joint leads to suppression of
proteoglycan synthesis and release of mediators responsible for degradation of
cartilage matrix components.
This is believed to be the cause of collapse or dimpling of the newly formed cartilage seen with prior art devices.
Furthermore, there is a dearth of knowledge about the
modes of mechanical
signal transduction in chondrocytes.
Thus, this places a limit of success for prior art devices having a matrix equal to, or less stiff than the surrounding
host tissue to 5 millimeters in
diameter.
However, any device having a cartilage scaffold matrix greater in stiffness than the surrounding
host tissue will not be properly influenced by mechanical
signal transduction and will either form calcified tissues or disorganized fibrocartilage that collapses as the matrix degrades and the tissue experiences stress loading.
Within the bone layer, known prior art devices failed to recognize the
impact a rigid scaffold has on the surrounding uninvolved tissue.
As this occurs, the bones lose minerals, heaviness (
mass), and structure, making them weaker and increasing their risk of collapse and or breaking.
Two functional problems identified with rigid porous scaffolds of prior art devices are as follows.
First these rigid devices do not contain elongated channels and thus they tend to dissipate and dampen the
hydrostatic pressure pulses that would normally flow through viscous fluids.
Secondly these devices are too rigid through the cartilage region thus not allowing for initial compression to establish a
pressure wave through the
bone marrow.
Concerning the
synovial fluid, prior art devices fail to recognize the role this substance plays in maintaining healthy articular cartilage.
 Login to View More
Login to View More