Group-iii element nitride crystal producing method and group-iii element nitride crystal
a technology of group-iii element and nitride crystal, which is applied in the direction of non-metal conductors, instruments, conductors, etc., can solve the problems of reduced growth rate and easy non-uniform nucleation, and achieve efficient growth of group-iii element nitride crystal, the effect of improving crystal growth rate and suppressing non-uniform nucleation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
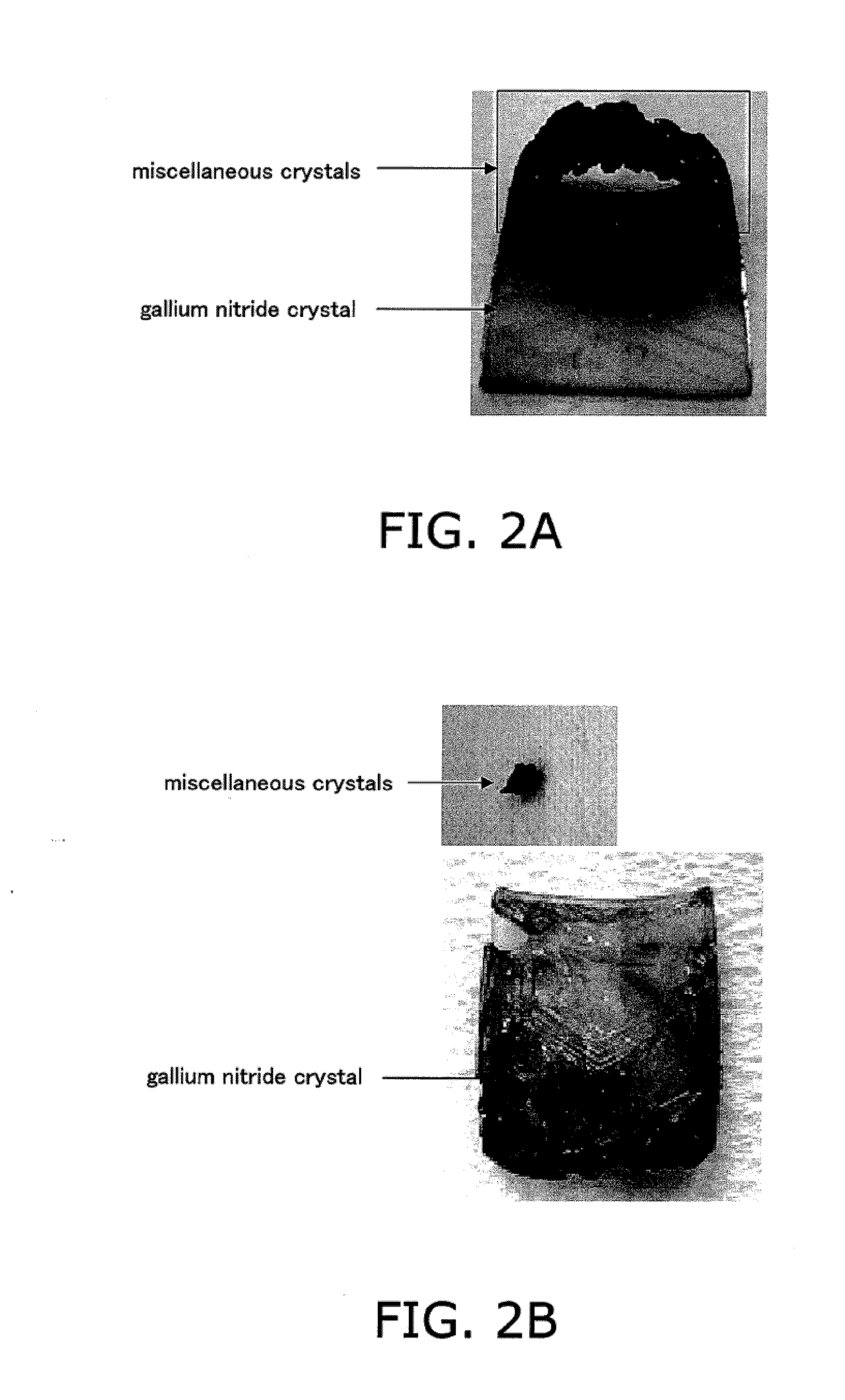
[0068] In this embodiment, a method for producing a gallium nitride crystal will be described in which a seed crystal, sodium, gallium, and a hydrocarbon are placed in a crystal growth vessel for holding a sodium flux, and thereafter, nitrogen-containing gas is pressurized and the crystal growth vessel is heated to melt sodium and gallium, thereby generating a sodium flux.
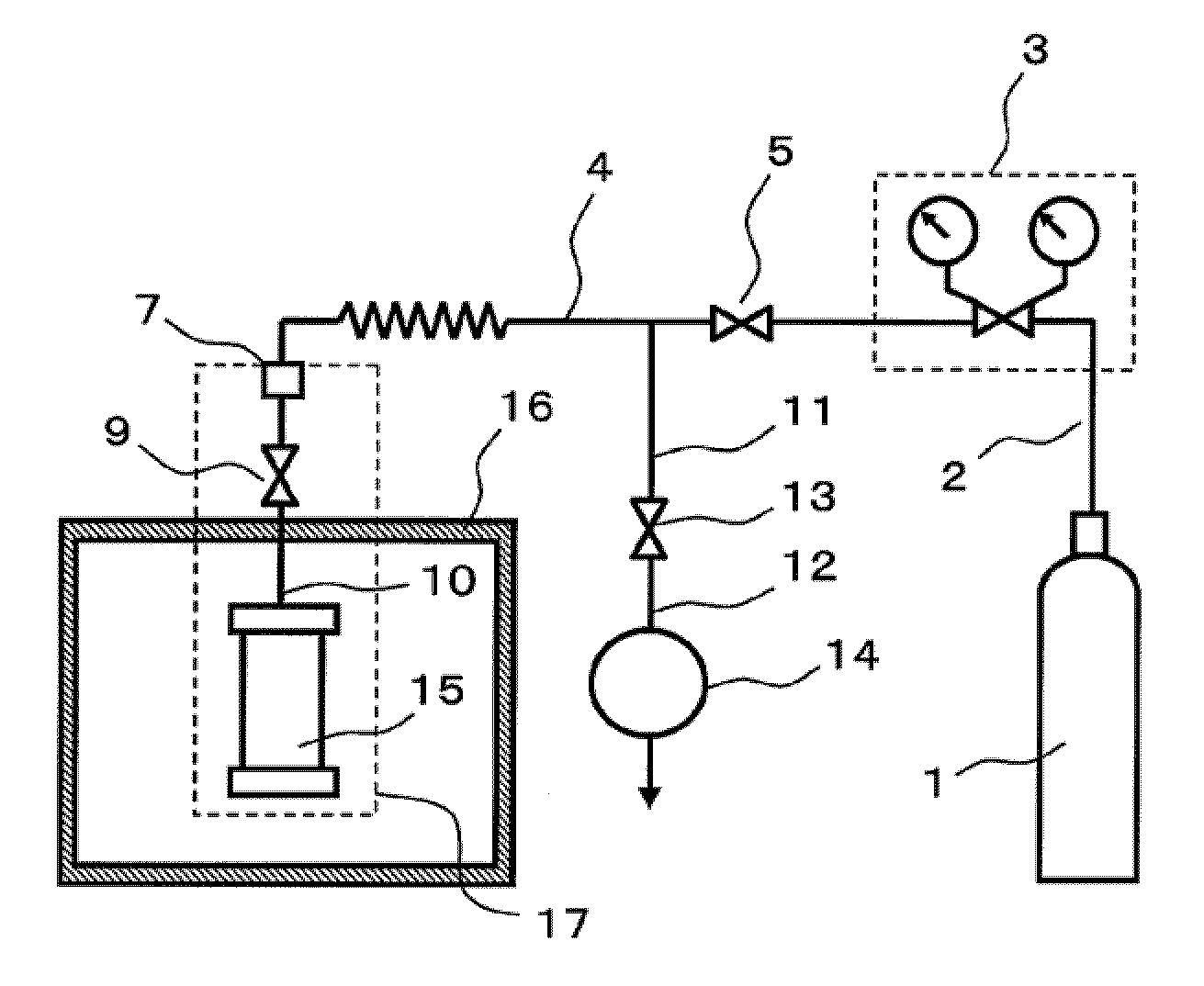
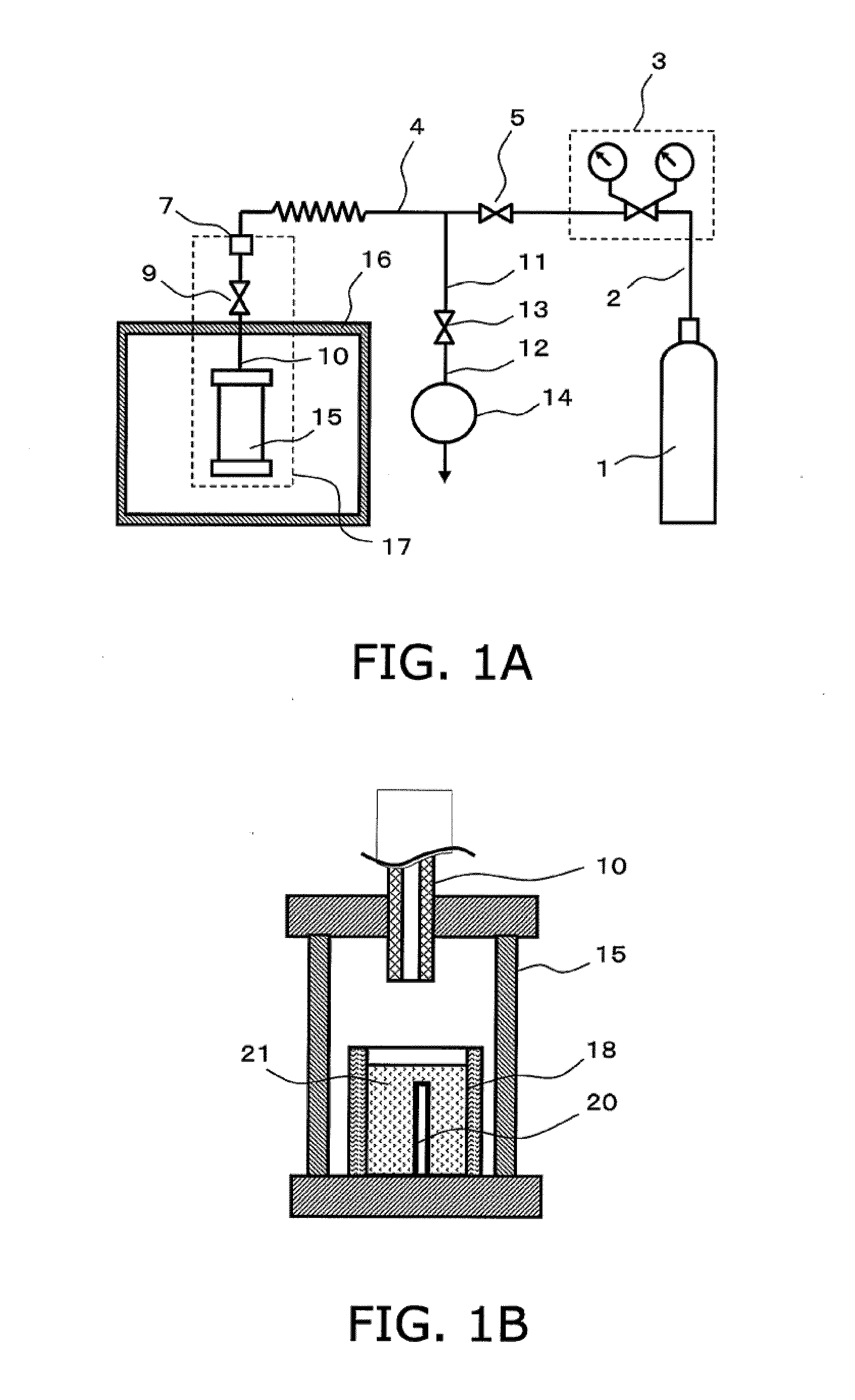
[0069] An exemplary configuration of a production apparatus that is employed in the production method of the present invention is shown in FIG. 1(a). An exemplary closed pressure-resistant and heat-resistant vessel for use in the production method of the present invention is shown in FIG. 1(b).
[0070] The apparatus of FIG. 1(a) comprises a gas supply apparatus 1 for supplying material gas, a pressure adjuster 3 for adjusting the pressure of the material gas, a closed pressure-resistant and heat-resistant vessel 15 for conducting crystal growth, a heating apparatus 16 for heating, and an exhaust apparatus 14.
[0071...
embodiment 2
[0093] In this embodiment, a method for producing gallium nitride will be described in which a seed crystal, sodium (alkali metal) coated with a hydrocarbon, gallium (group-III element), and a hydrocarbon are placed in a crystal growth vessel for holding a sodium flux that is an alkali metal, and thereafter, a portion of the hydrocarbon is removed, followed by heating of the crystal growth vessel.
[0094] In this production method, further, the amount of the hydrocarbon in the crystal growth vessel is preferably adjusted. For example, the suppression of nucleation and the coating of the alkali metal require different amounts of the hydrocarbon. Therefore, the hydrocarbon may be added in amount required for the coating of the alkali metal in a preliminary step, and the amount of the hydrocarbon may be adjusted within an appropriate range so as to prevent dissolution of the seed crystal in an adjustment step. The adjustment of the amount of the hydrocarbon includes, for example, remova...
embodiment 3
[0102] A group-III element nitride crystal of the present invention is produced by the production method of the present invention described above.
[0103] This crystal preferably has an optical absorption coefficient of 10 cm−1 or less with respect to light having a wavelength of 400 nm or more and 620 nm or less. The optical absorption coefficient is preferably 5 cm−1 or less. Note that the lower limit of the optical absorption coefficient is a value exceeding zero.
[0104] The group-III element nitride crystal produced by the production method of the present invention may contain carbon. For example, the group-III element nitride crystal of the present invention may contain 5×1017 (cm−3) or less carbon atoms as a result of analysis by SIMS or the like.
[0105] In such a crystal, the group-III element is at least one element selected from Al, Ga and In. The group-III element nitride is preferably a compound that is represented by AlsGatIn(1-s-t)N, where 0≦s≦1, 0≦t≦1, and s+t≦1.
[0106]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical absorption coefficient | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com