Fuel additive for enhancing combustion efficiency and decreasing emissions
a technology of additives and fuels, applied in the direction of fuel additives, liquid carbonaceous fuels, fuels, etc., can solve the problems of increasing greenhouse gases into the atmosphere, acid rain is toxic to both animals and plants, and the jet engine or heating furnace is a hazard to the ecosystem of the world, so as to reduce the emissions of toxic exhaust and increase the efficiency of burn
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
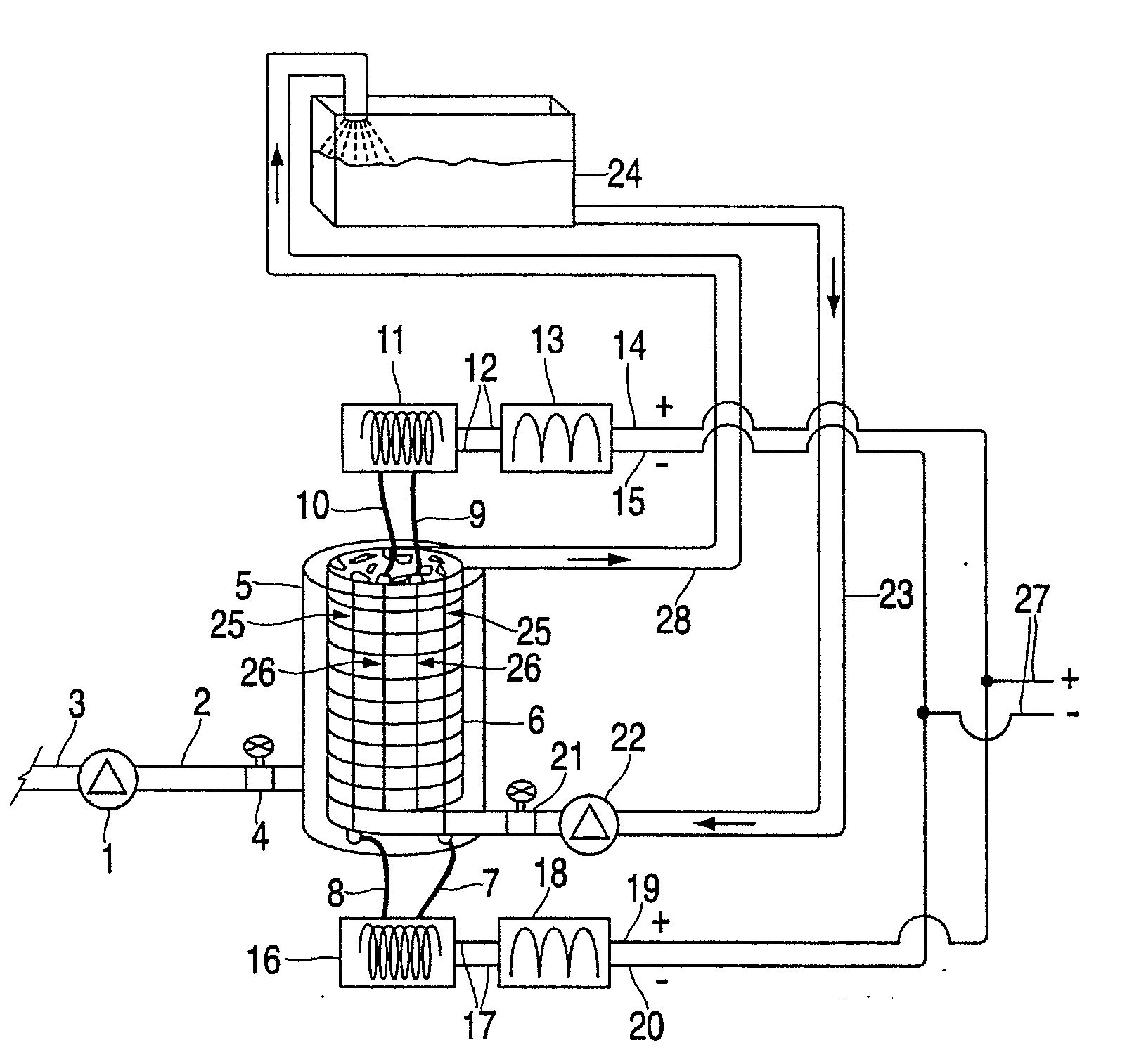
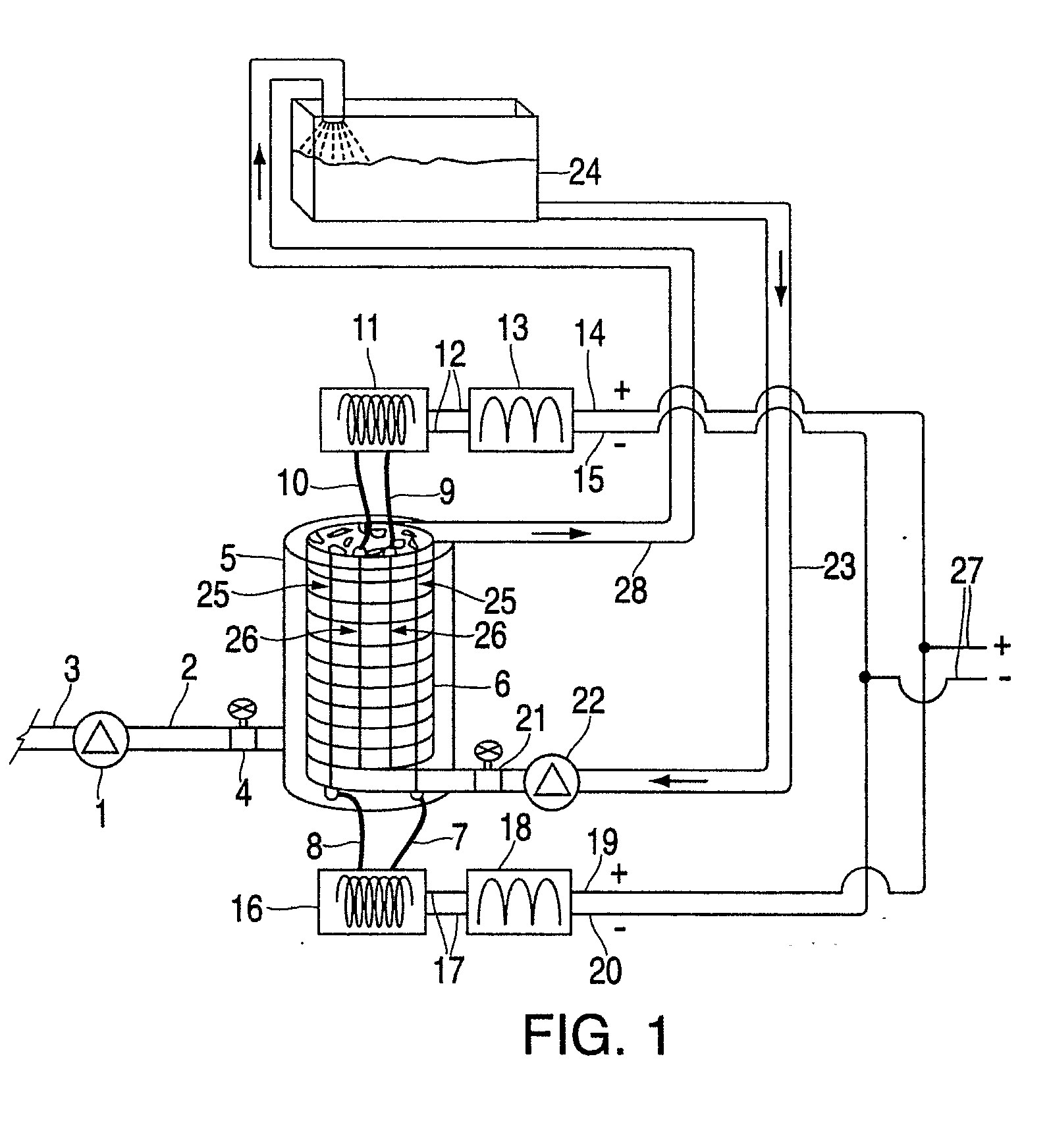
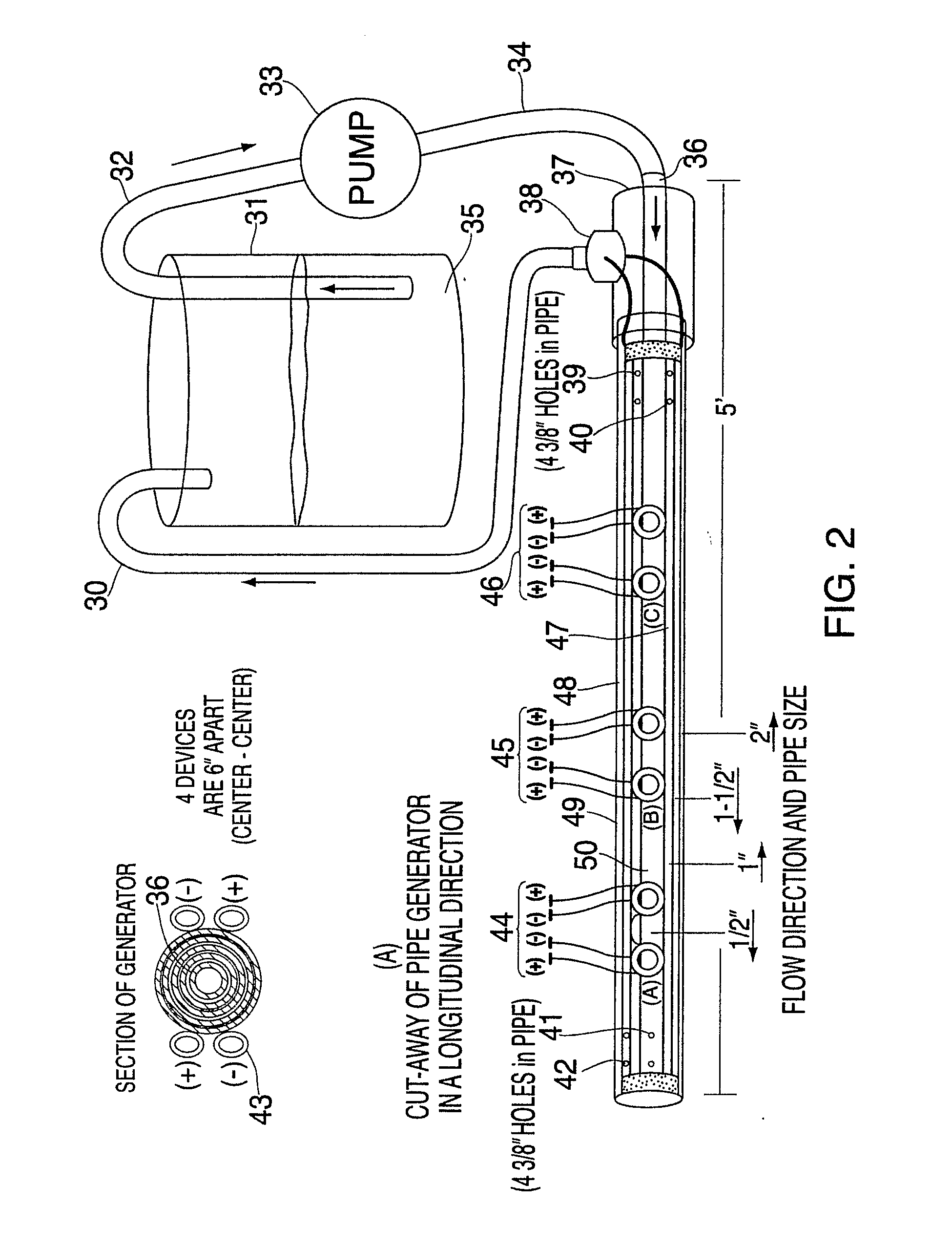
[0076]Without limiting the invention to specific theories of operation or to the specific embodiments disclosed herein, the inventors' preferred embodiments, as well as the inventors' present understanding of the theory of operation, will now be described.
[0077]With respect to the active moiety of the fuel additive, it is the inventors' belief that the aqueous colloid, such as a silica colloid, is a processing aide and a carrier to the combustion chamber wall such that adhesion occurs through an electrostatic charge on the palladium silicate, palladium silicide and palladium acetate bound to the silica colloid of the invention. The palladium silicate colloid complex is moderately soluble in kerosene and soluble in pH 4.35 aqueous (partition coefficient ˜1 / 10). PdO is insoluble in aqueous at pH 4.35 and kerosene. Palladium (II) acetate is insoluble in water and at least substantially insoluble in organic, but is soluble in acetic acid.
[0078]In the practice of the preferred method of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com