Treatment of disease with poly-n-acetylglucosamine nanofibers
a technology of polyn-acetylglucosamine and nanofibers, which is applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., to achieve the effect of increasing the metabolic rate of serum-starved human umbilical cord, and increasing the metabolic rate of serum-starved human cord blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6.1 Example 1
sNAG Nanofibers from a Marine Diatom Promote Wound Healing and Defensin Expression Via an Akt1 / Ets1-Dependent Pathway
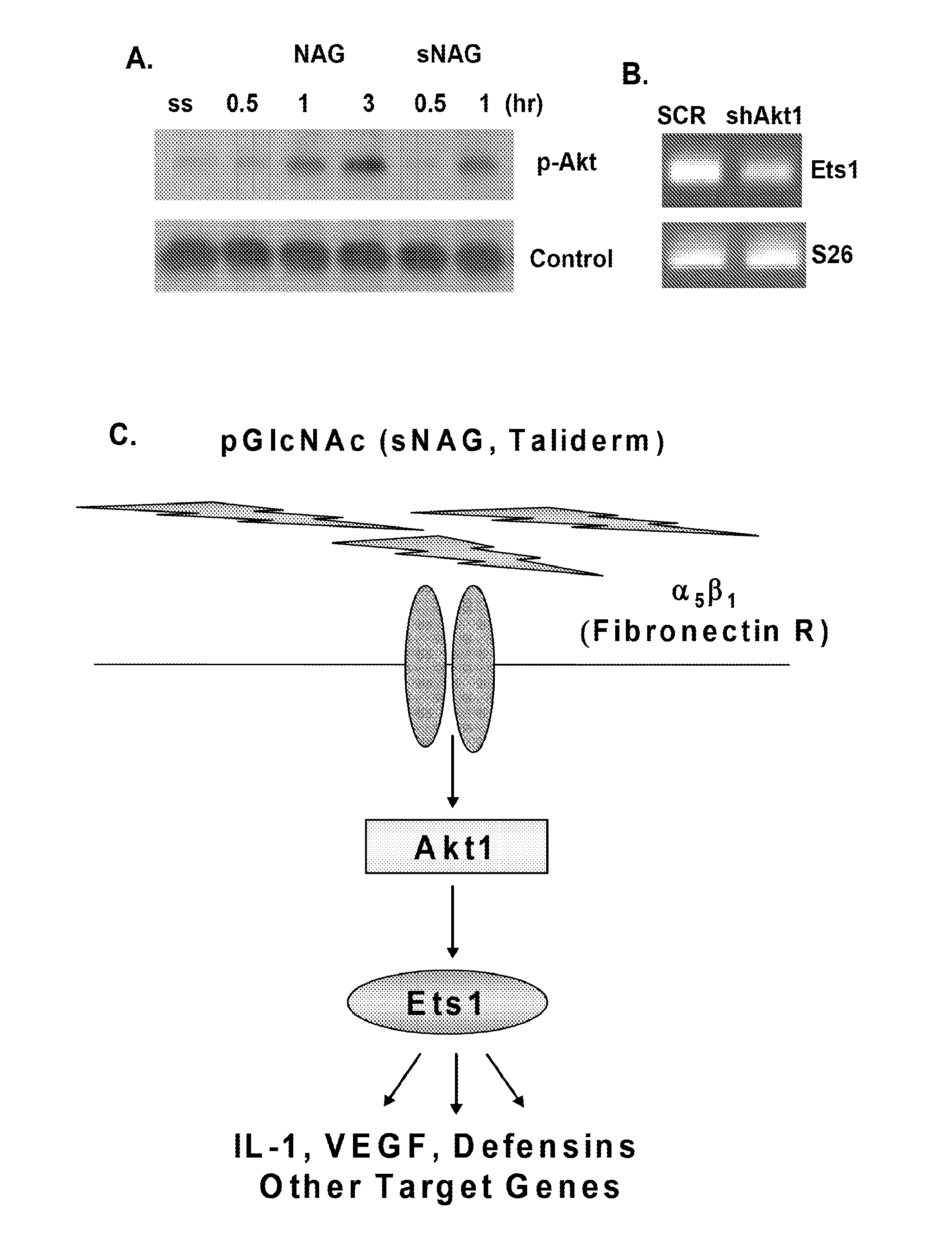
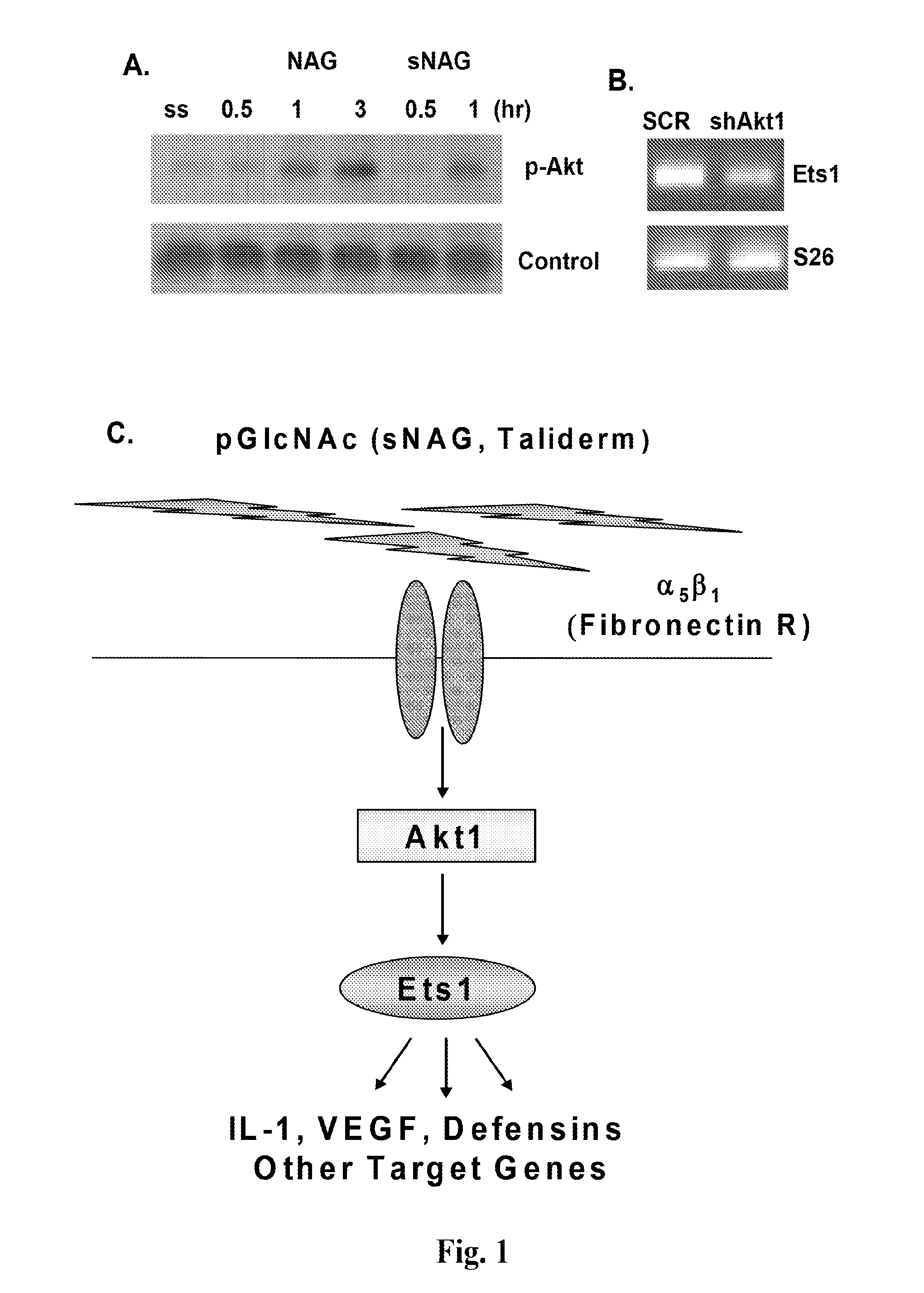
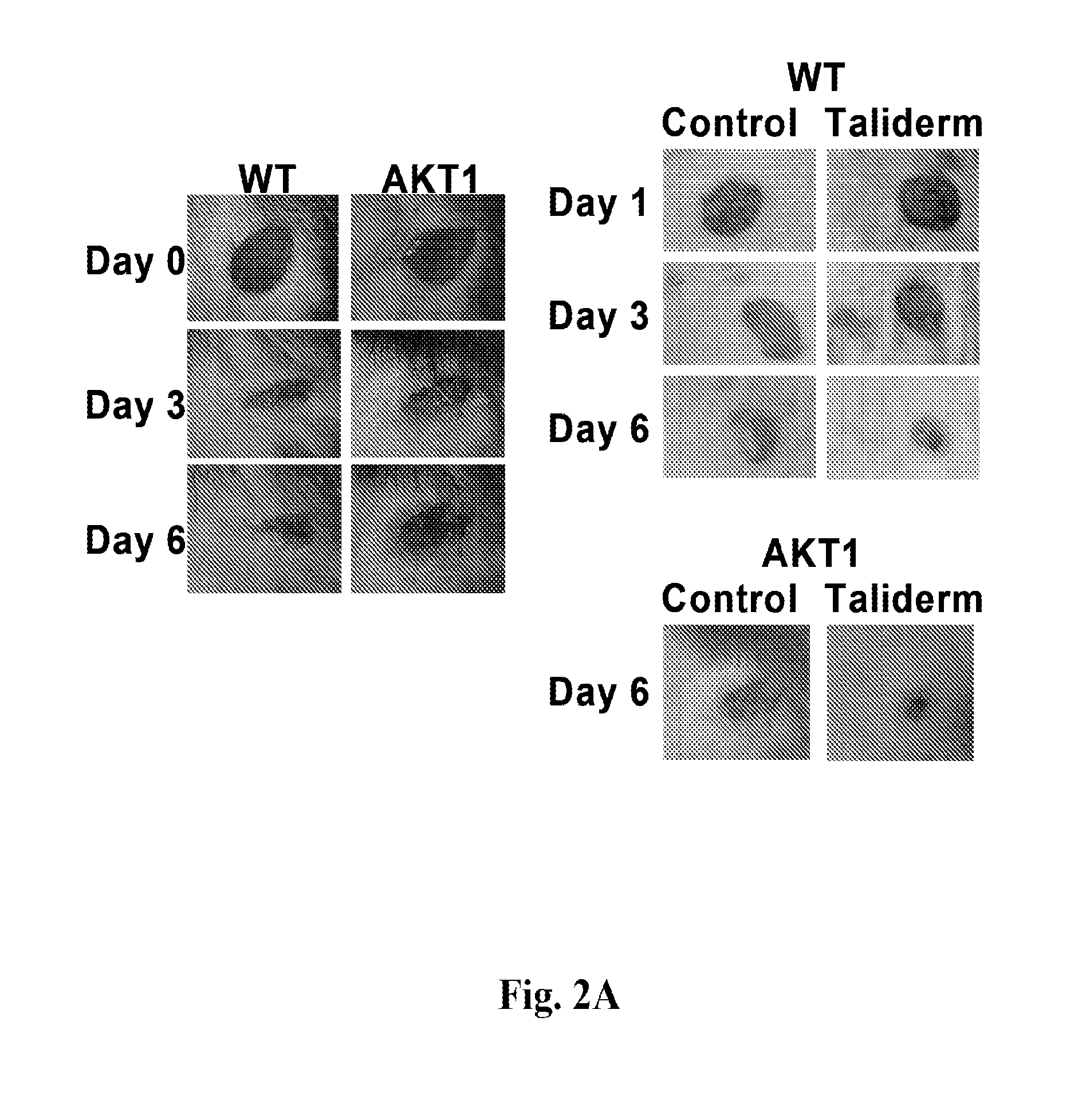
[0228]This example demonstrates that sNAG nanofibers promote cutaneous wound healing and expression of defensins, and that the Akt1→Ets1 pathway plays a central role in the regulation of cutaneous wound healing by sNAG nanofibers.
6.1.1 Materials and Methods
[0229]sNAG nanofibers (in particular, Taliderm) are produced and supplied by Marine Polymer Technologies and formed into suitable patches for wound treatment. Wildtype C57 Black and Akt1 null mice were housed at the Medical University of South Carolina animal facilities. Wildtype and Akt1 null mice, ages ranging from eight to 12 weeks, were anesthetized with 50% pure oxygen and 50% isoflurane gas. Immediately before wounding, Nair Hair Removal Lotion was applied to their dorsum to remove any unwanted hair. A dorsal 4 mm circular area of skin was removed using an excision biopsy punch. Taliderm was place...
example 2
6.2 Example 2
sNAG Nanofibers Increase Defensin Expression, Increase Kinetics of Wound Closure, and have an Indirect Defensin-Dependent Anti-Bacterial Effect
[0250]This example demonstrates that sNAG nanofibers have a potent anti-bacterial effect against Staphylococcus aureus in vivo, which is indirect and defensin-dependent. This example also shows that sNAG nanofibers induce expression of defensins in vitro in keratinocytes and endothelial cells and in vivo in cutaneous wounds, in an Akt-1 dependent manner, and increase the kinetics of wound closure.
6.2.1 Materials and Methods
[0251]Tissue Culture, Pharmacological Inhibition, ELISA:
[0252]Human umbilical cord vein EC (Lonza) were maintained at 37° with 5% CO2 in endothelial basal medium 2 (Lonza). Endothelial basal medium 2 (EBM2) was supplemented with EC growth medium 2 SingleQuots as described by Lonza procedures and 1% penicillin / streptomycin (Invitrogen). Serum starvation was performed at 80-90% confluency in EBM2 supplemented wit...
example 3
6.3 Example 3
sNAG Nanofibers Upregulate Expression of a Number of Defensins and Toll Receptor Genes
[0299]This example demonstrates that a number of defensins and Toll-like receptors are up-regulated by sNAG treatment of human endothelial cells.
[0300]Materials and Methods:
[0301]Human Chip probes were printed on epoxy slides. HUVEC cells were cultured as described in section 6.2, and treated with sNAG nanofibers (“sNAG”) for 5 hours. RNA was extracted with RNAsol (Teltest, Inc.) following manufacturer's instructions, amplified using Amino Allyl MessageAMP™ II aRNA amplification kit (Applied Biosystems), and labeled. The slides were prepared for hybridization with aRNA by soaking in blocking solution (Sigma Tris-buffered saline pH8.0, in 1000 ml dH2O, 1% BSAw / v, NaN3 to 0.05%) at RT O / N, then rinsed and dryed. Samples containing labeled target aRNA from sNAG-treated cells were hybridized with the slides (65 ul / slide; denatured at 95° C. for 5 min; hybridized for 48 hours at 37° C. in 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com