Compositions and methods for the separation of metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
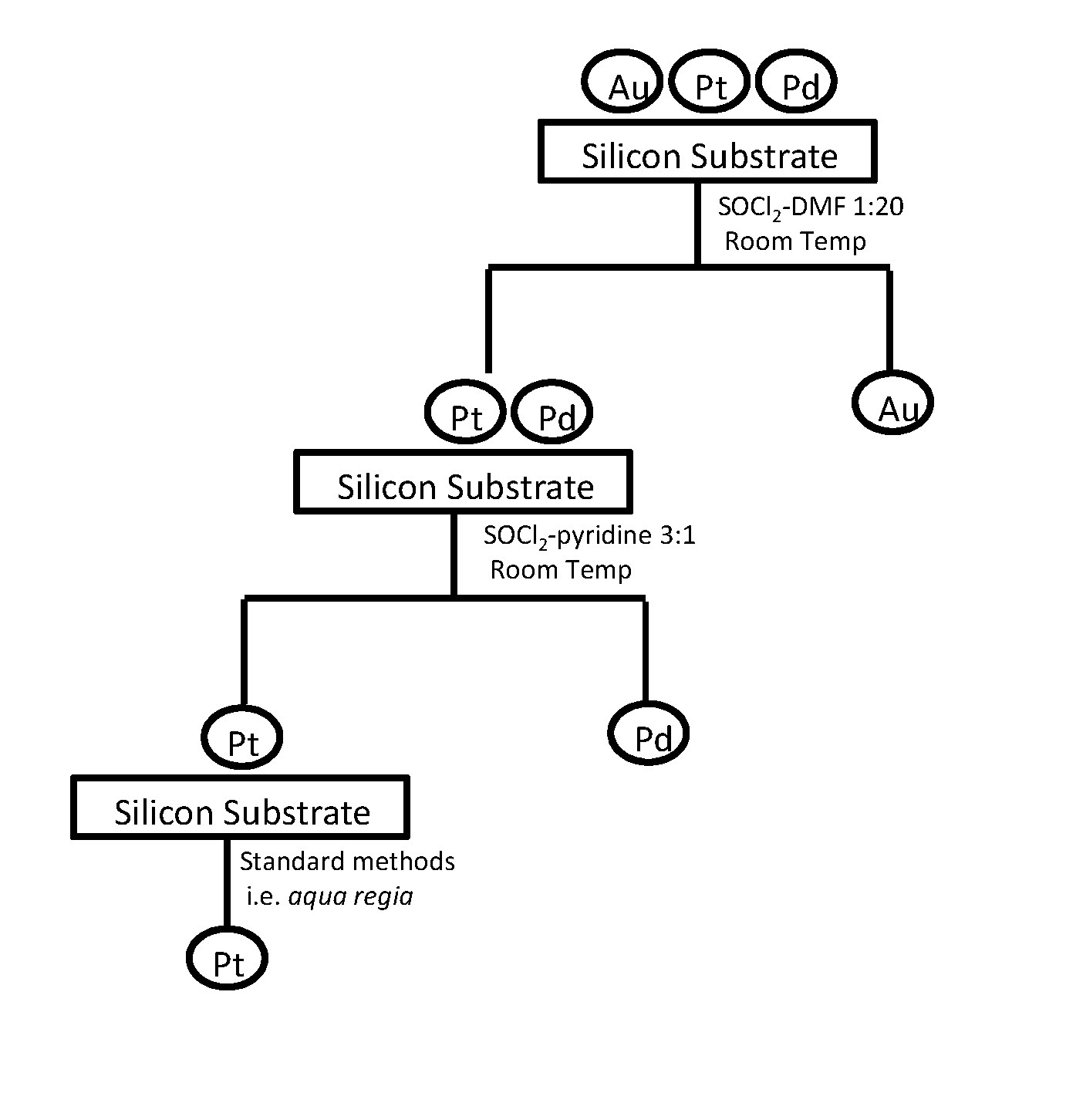
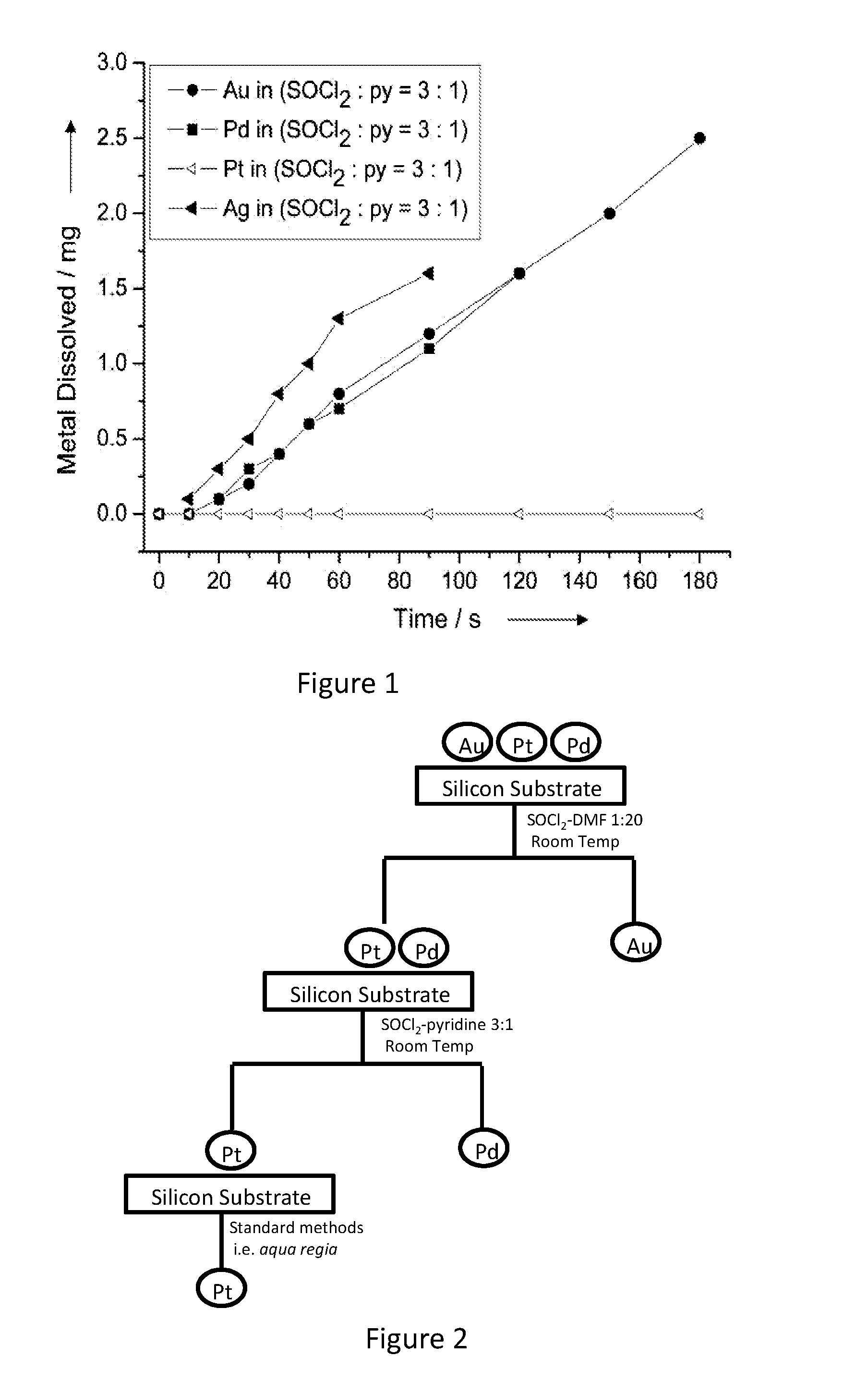
[0051]Layers of 250-nm-thick Au, Pd, Ag, and Pt, was deposited, onto a 9-cm2 silicon substrate each, with 20-nm-thick chromium as the adhesion layer (chromium was not soluble in any reaction medium) to avoid the error that might be introduced due to peel-off of the noble metal metallization layer. The metalized substrates were immersed in 20 mL of 3:1 v / v SOCl2-py at room temperature with mild shaking for a preset duration, taken out, rinsed thoroughly, dried, and weighed. No weight loss was detected of a platinum film after it was immersed in the SOCl2-py mixture at room temperature or even at 70° C. (refluxing) for 1 week.
[0052]FIG. 1 shows the kinetic results of the dissolution of noble metals in the mixture of SOCl2 and pyridine (py) with a volumetric ratio of 3:1. SOCl2 dissolved Au at a rate of 0.3 mol m−2 h−1 at room temperature, which is faster than Au dissolution in conventional cyanide leaching agents (−2 h−1) and iodide solutions (−2 h−1). Silver (Ag) and Pd also dissolve...
example 2
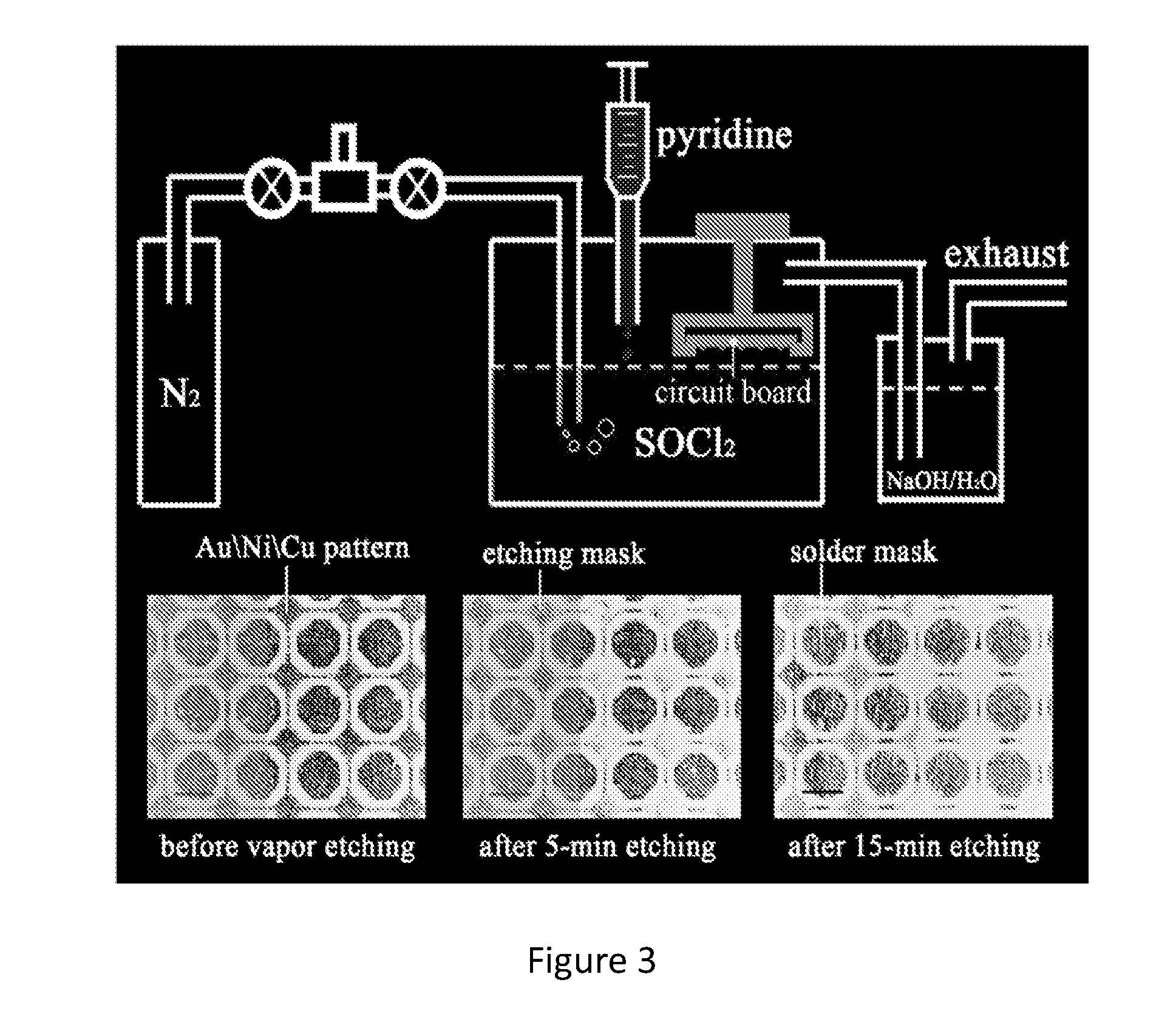
[0053]Different solutions in the method were analyzed by different techniques. X-ray photoelectron spectroscopy of the reaction of gold in SOCl2-py showed the Au 4f7 / 2 binding energy at 86.6 eV characteristic of the oxidation state of Au(III). To demonstrate that Au was oxidized to Au(III) instantaneously, SOCl2-pyridine vapor was used to etch an Au / Si surface. Au(III) was confirmed on the vapor-etched Au / Si surface by XPS and Raman spectroscopy in the form of AuCl4−. Vibration modes of Au(III)-Cl, however, were not observed in the Raman spectrum probably because they were covered by the strong fluorescence background. Actually, after the 0.03 mol L−1 Au / 3:1 SOCl2-py solution was stored at room temperature in a dark room for 150 days, a large amount of yellow precipitate was observed and the solution turned into a dark suspension. [AuCl4]− was confirmed in the Raman spectrum of the precipitate. Therefore, [AuCl4]− appeared to form as a result of Au dissolution in SOCl2-py mixtures. ...
example 3
[0057]Gold nanoparticles were synthesized by reducing 12 mg HAuCl4.3H2O in 10 mL oleylamine at 105° C. for 2 h under an Ar blanket, collected by precipitation with ethanol and centrifugation, and washed with mixtures of acetone and hexane. Platinum nanoparticles were synthesized by reducing 30 mg H2PtCl6.6H2O in 20 mL ethylene glycol (containing 35 mg polyvinylpyrrolidone, Mw=29000) at 165° C. for 2 h under an Ar blanket, collected by precipitation with acetone and centrifugation, and washed with mixtures of acetone and ethanol [17].
[0058]20 mg dried gold nanoparticles and 5 mg dried platinum nanoparticles were mixed, and then added into 10 mL SOCl2 to form a dark brown mixture. A certain amount of pyridine was added at room temperature, changing the colour of the mixture to light brown. The solution was diluted into 200 mL acetonitrile. The dissolved Au went into the solution; the non-dissolved nanoparticles were precipitated out of the solution, washed, and collected. The yield wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com