Reductive electroless gold plating solution, and electroless gold plating method using the plating solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
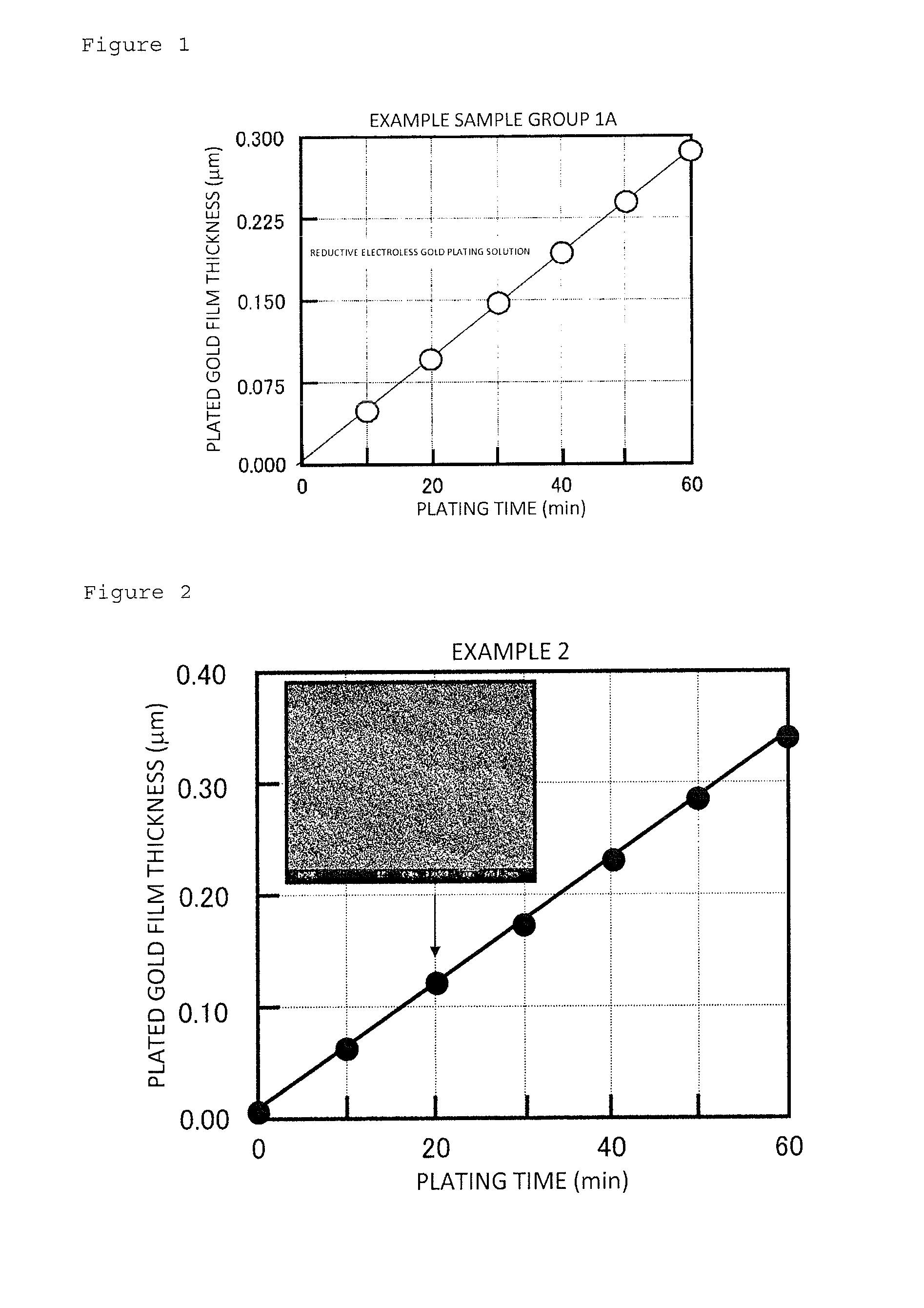
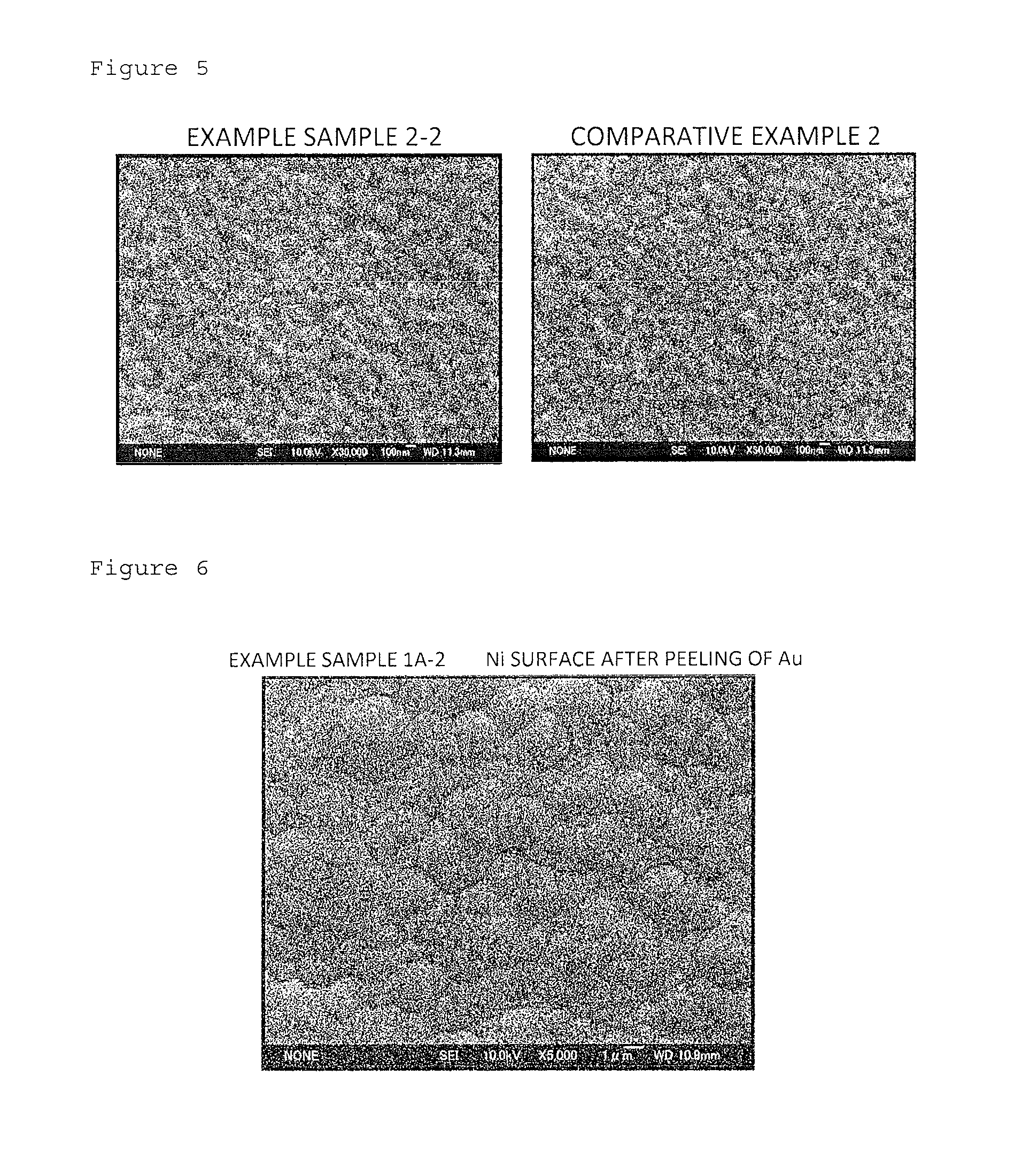
[0063]In Example 1, by using a reductive electroless gold plating solution to which the present invention was applied and using a copper plate as a substrate, plated films composed of an electroless plated nickel film / electroless plated palladium film / electroless plated gold film were formed on the substrate.
Preparation of the reductive electroless gold plating solution: The composition of the reductive electroless gold plating solution used in the present Example is shown in the below. The plating condition (pH, solution temperature) is shown together with the composition.
[0064]Potassium gold cyanide: 5 mmol / L
[0065]Dipotassium ethylenediaminetetraacetate: 0.03 mol / L
[0066]Citric acid: 0.15 mol / L
[0067]Hexamethylenetetramine: 3 mmol / L
[0068]3,3′-diamino-N-methyldipropylamine: 0.02 mol / L
[0069]Thallium acetate: 5 mg / L
[0070]pH: 8.5
[0071]Solution temperature: 80° C.
Fabrication of plated films: Samples with a plated film as Example 1 were composed of an Example sample group 1A to an Example...
example 2
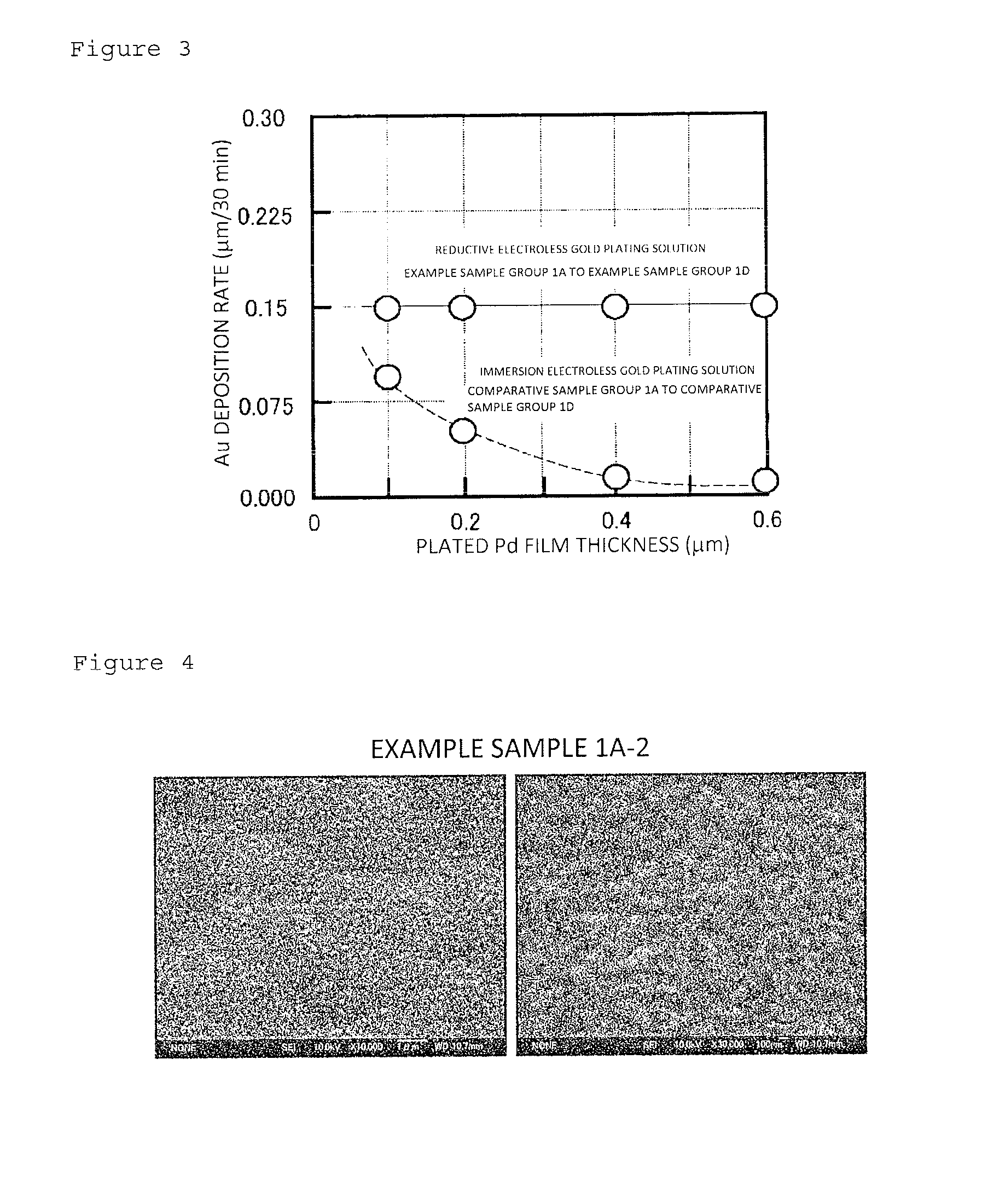
[0076]In Example 2, by using the reductive electroless gold plating solution as in Example 1 and using a copper plate as a substrate, plated films composed of an electroless plated nickel film / immersion electroless plated gold film / reductive electroless plated gold film were formed on the substrate. Samples with a plated film as Example 2 were composed of an Example sample 2-1 to an Example sample 2-6. The Example sample 2-1 to the Example sample 2-6 were each made by forming an electroless plated nickel film of 5 μm in film thickness on the surface of the copper plate, and thereafter forming an immersion electroless plated gold film of 0.07 μm in film thickness on the surface of the electroless plated nickel film. Thereafter, a reductive electroless plated gold film was formed on the surface of the immersion electroless plated gold film by using the above-mentioned reductive electroless gold plating solution according to the condition of a corresponding plating time. Here, in each ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com