Method for preparation of cellulose nanocrystals and nanofibrils
a technology of nanofibrils and nanocrystals, which is applied in the field of preparation of cellulose nanocrystals and nanofibrils, can solve the problems of large amount of acidic waste, high cost, and general harsh conditions of hydrolysis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047]Preparation of Oxidized Cellulose Nanofibrils Without TEMPO
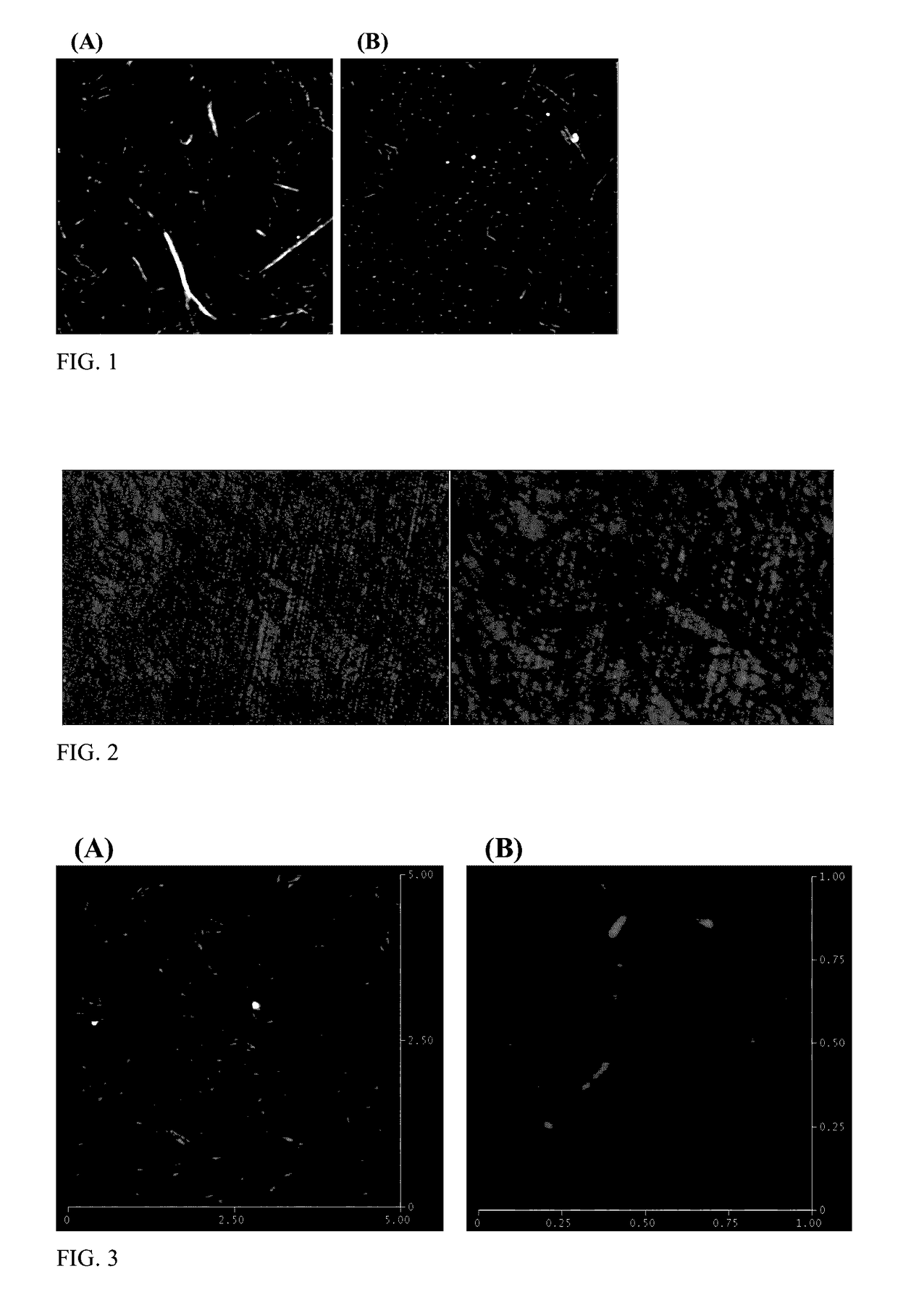
[0048]A solution of mechanically treated cellulose pulp (37.5 grams, 2.67% solid content) was dispersed in water (62.5 milliliters) containing sodium bromide (0.1 grams). A solution of sodium hypochlorite (2.1 grams, active chlorine 10-15%) was added to the suspension. Sodium hydroxide (5.5 grams, 0.5 M in water) was introduced to the mixture to adjust pH to about 12. The reaction mixture was stirred for 20 hours at room temperature, then centrifuged at 4000 rpm for 15 minutes. The supernatant was discarded, and the precipitate was redispersed in water and centrifuged to remove the remaining reagents. The redispersion and centrifugation was repeated three times until the pH of supernatant is close to DI water (about 6). After centrifuging, the precipitate was isolated, dispersed in 80 milliliters of water, and sonicated for 50 minutes to disintegrate the nanocellulose into single nanofibrils. The nanofibril solution wa...
example 2
[0050]Preparation of Oxidized Nanocellulose With TEMPO
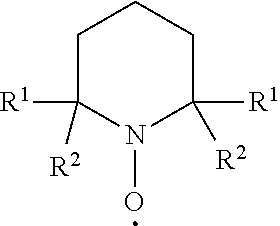
[0051]A solution of mechanically treated cellulose pulp (37.5 grams, 2.67% solid content) was dispersed in water (62.5 milliliters) containing sodium bromide (0.1 grams) and 2,2,6,6-tetramethyl-1-piperidine-N-oxy (TEMPO, 0.016 grams). A solution of sodium hypochlorite (2.1 grams, active chlorine 10-15%) was added to the suspension. Sodium hydroxide (5.5 grams, 0.5 M in water) was introduced to the mixture to adjust pH to 12. The reaction mixture was stirred for 20 hours at room temperature, then centrifuged at 4000 rpm for 15 minutes.
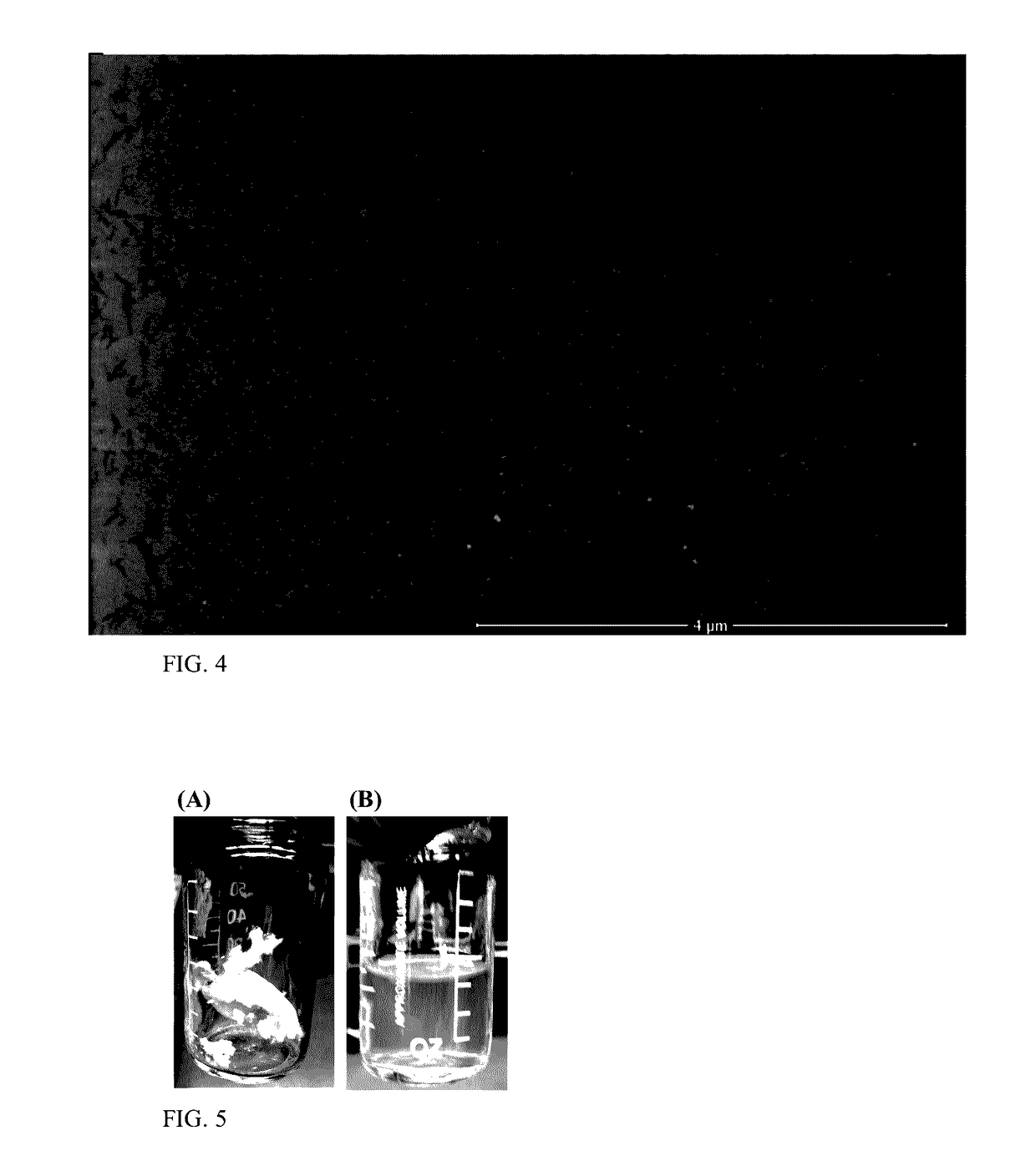
[0052]A cellulose nanocrystal colloidal solution was isolated as the transparent supernatant following centrifugation. The colloidal solution was precipitated in acetone and washed with ethanol or methanol three times to remove residual salts and TEMPO. The precipitated cellulose nanocrystals were dried at room temperature under vacuum for 24 hours. The cellulose nanocrystals were observed to have a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com