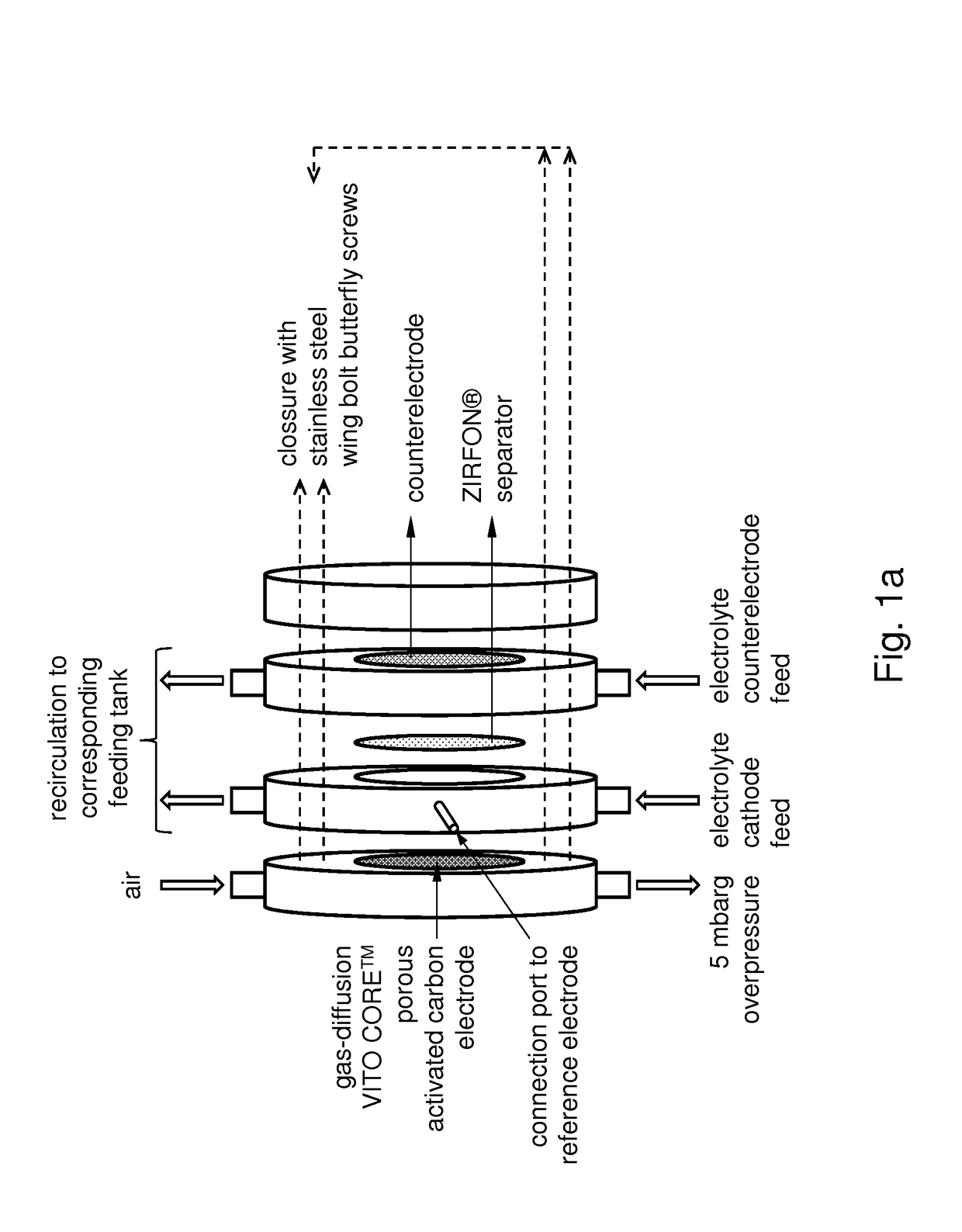
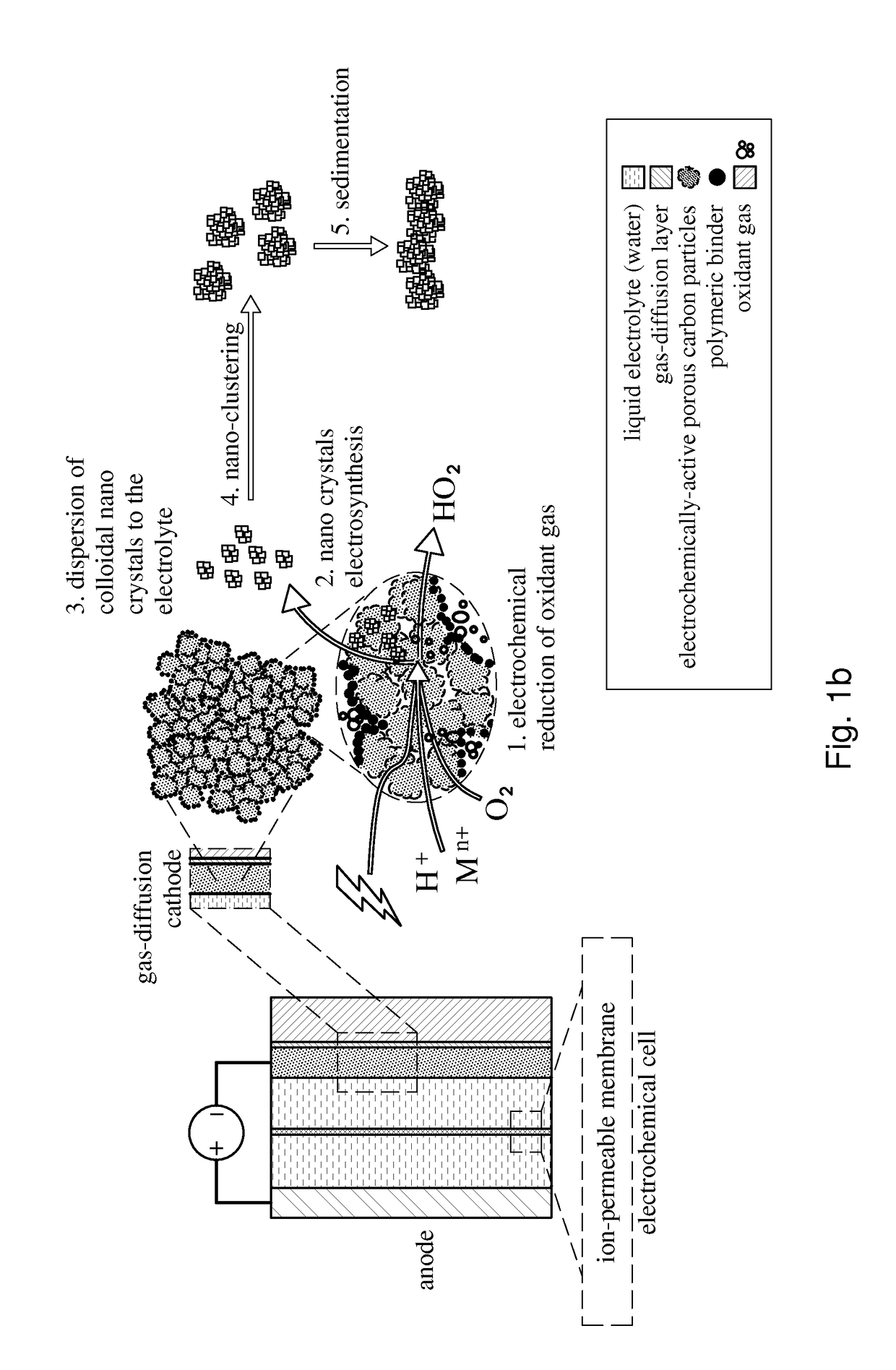
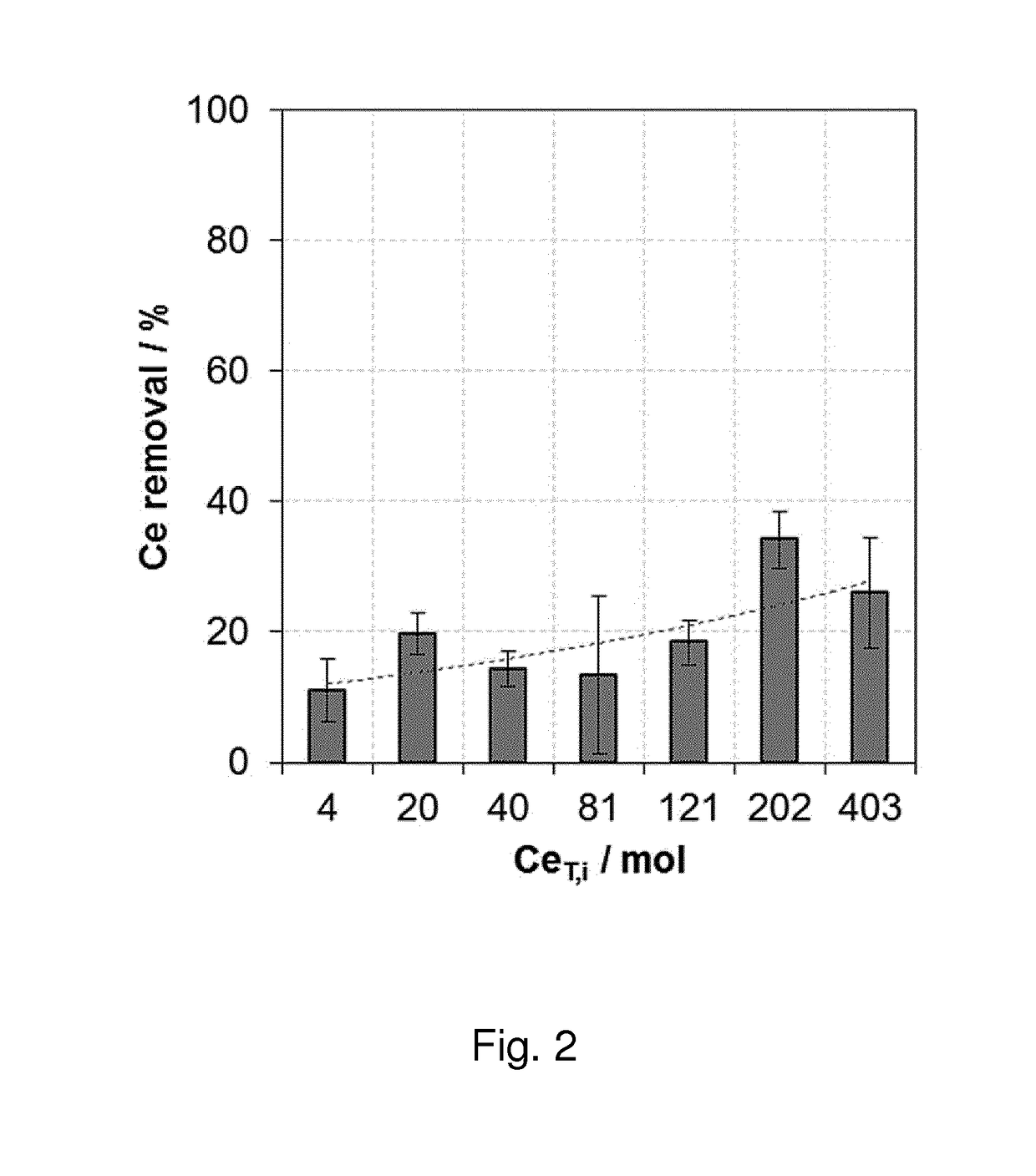
An electrochemical process for preparing a compound comprising a metal or metalloid and a peroxide, ionic or radical species
a technology of peroxide and compound, applied in the direction of electrolysis process, electrode, electrolysis components, etc., can solve the problems of unsatisfactory ree demand, high polydisperse size and shape distribution, and the above described methods do not provide true, economically feasible recovery rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0201]Independent electrodes were tested as gas-diffusion cathodes, in presence of air at the gas compartment (to provide O2 for its reduction to H2O2, its polyatomic ions or radical). The reagents presented in Table A were dissolved in demineralized water and the pH of the solution where the pH was adjusted to approximately 4.
TABLE AComposition of catholyte in demineralized water.ChemicalQuantityChemical nameformula(mg · L−1)1Cerium nitrate hexahydrateCe(NO3)3•6H2O3502Dysprosium nitrate x hydrateDy(NO3)3•xH2O803Erbium nitrate pentahydrateEr(NO3)3•5H2O534Europium nitrate pentahydateEu(NO3)3•5H2O65Gadolinium nitrateGd(NO3)3•6H2O59hexahydrate6Holmium nitrateHo(NO3)3•5H2O18pentahydrate7Lanthanum nitrateLa(NO3)3•6H2O159hexahydrate8Lutetium nitrate hydrateLu(NO3)3•xH2O69Neodymium nitrateNd(NO3)3•6H2O206hexahydrate10Praseodymium nitratePr(NO3)3•6H2O51hexahydrate11Samarium nitrateSm(NO3)3•6H2O53hexahydrate12Terbium nitrate hexahydrateTb(NO3)3•6H2O1213Thulium nitrate pentahydrateTm(NO3)3•5H...
example 3
[0207]The composition of the electrolyte was identical to that explained for example 1, but lanthanum nitrate was used instead of cerium nitrate, in concentrations of 0 ppm, 100 ppm, 500 ppm, 1000 ppm and 5000 ppm.
[0208]The initial pH and conductivity of the catholytes containing the different concentrations of the metal are disclosed in Table b. The operational volume of the catholyte in each experiment was 125 mL.
TABLE bMeasured pH and conductivity of the catholyteswith different concentrations of lanthanum nitrateLa(NO3)3•6H2O by the start of experimentation.Concentration (ppm)010050010005000CatholytepH2.542.152.702.782.76Conductivity51.051.650.450.352(mS · cm−1)AnolytepH2.82.82.742.742.74Conductivity49.749.749.849.849.8(mS · cm−1)
[0209]The concentration of lanthanum was quantitatively analyzed by means of ICP-MS.
[0210]A colourless solution was formed when dissolving the chemicals. Air was supplied to the gas compartment. After 2 h of processing at constant polarization condition...
example 4
[0211]The composition of the electrolyte was identical to that explained for example 1 but instead of cerium nitrate a boric acid was supplied in the catholyte. The concentration of boric acid was kept constant for all experiments (5 g·L−1). The effect of the polarization potential was evaluated. The following potentials vs. the reference electrode were compared: −0.350 V, −0.550 V, −0.750 V, −0.950 V. The operational volume of the catholyte in each experiment was 125 mL.
TABLE DpH and conductivity of the catholyte at the startof experimentation at different cathode potentials.Applied potential (V vs Ag / AgCl 3M KCl)−0.150−0.350−0.550−0.750−0.950CatholytepH2.672.632.512.512.76Conductivity47.147.447.247.446.9(mS · cm−1)AnolytepH2.742.742.82.82.8Conductivity49.849.849.749.749.7(mS · cm−1)
[0212]From the changes in the pH and conductivity, especially of the catholyte, it can be observed that the same trend found in previous examples was observed; this is, the pH significantly increased th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com