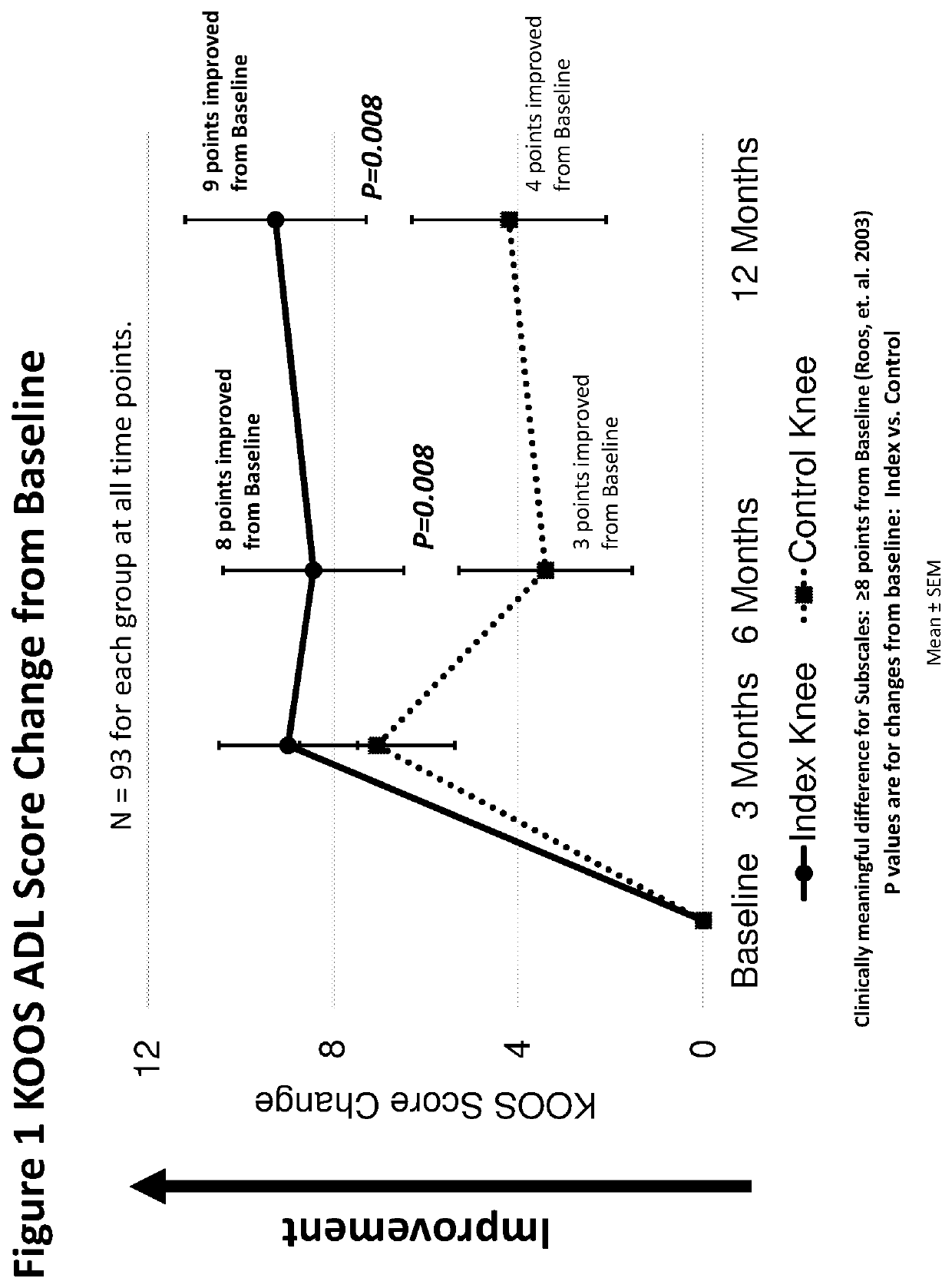
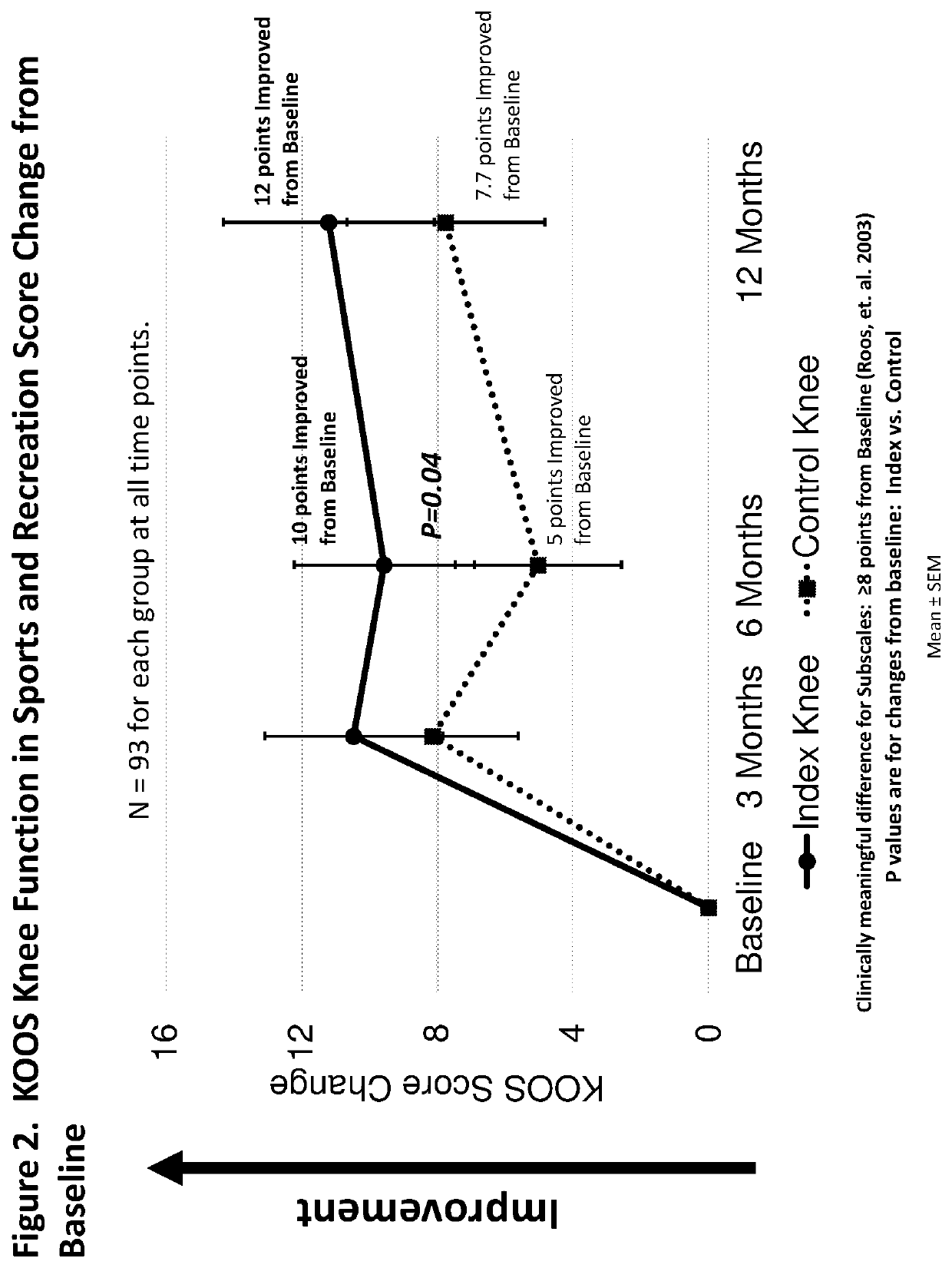
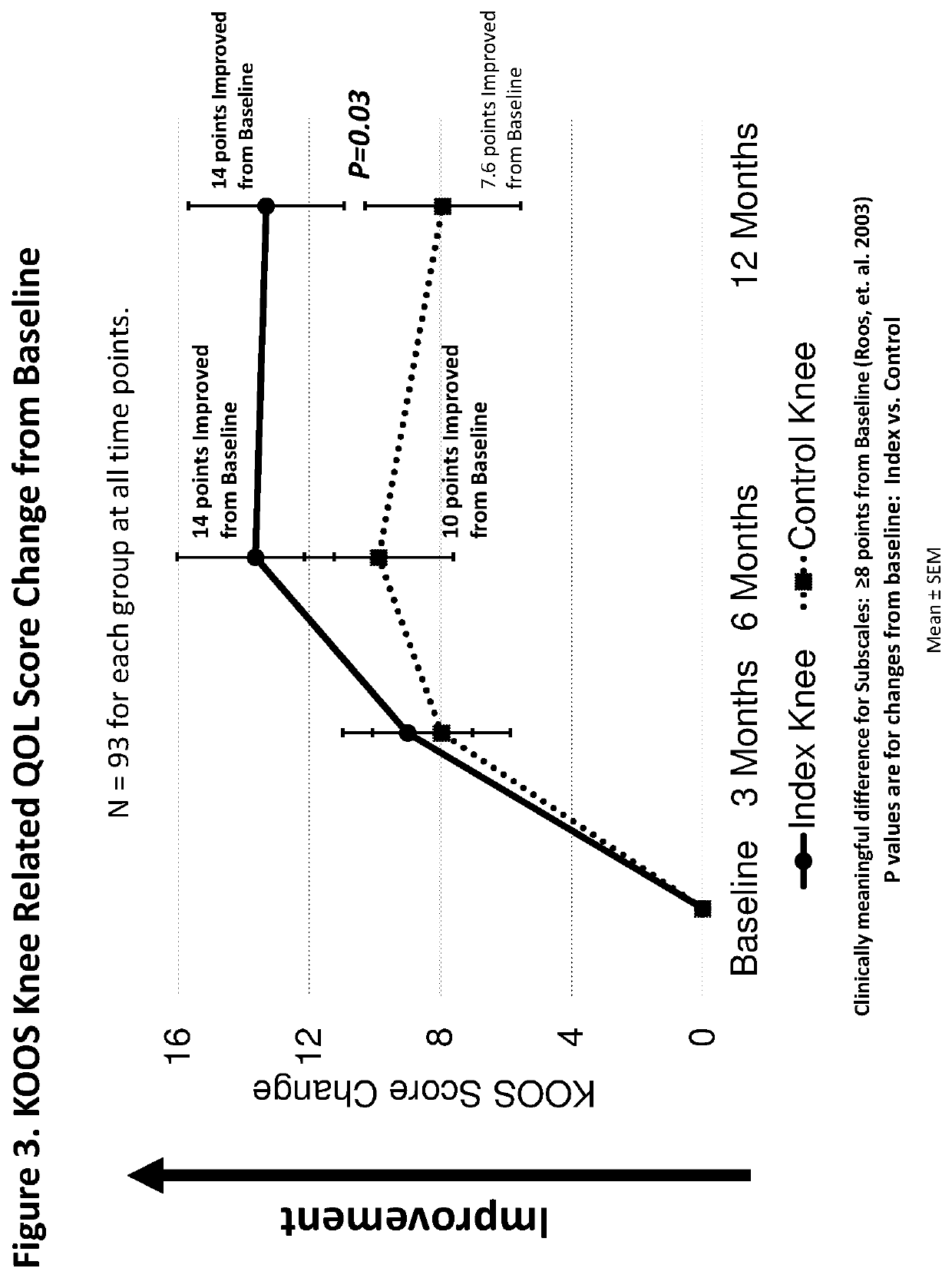
Method of treating osteoarthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Randomized Double-Blind Placebo Control Study of TPX-100 in the Patients with Osteoarthritis of the Knees
[0129]Clinical Study Methodology
[0130]Outline of the Study
[0131]A multicenter, randomized double-blind, placebo-controlled study was designed to investigate the safety, tolerability, pharmacokinetics, and efficacy of TPX-100 administered in four weekly doses in subjects with bilateral patello-femoral knee osteoarthritis. The study was conducted under an open IND (investigational new drug application) at CDER (Center for Drug Evaluation and Research) of the U.S. FDA (The United States Food and Drug Administration) in compliance with GCP (Good Clinical Practice) and ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) guidelines. Eighteen (18) orthopedic, rheumatologic and family practice centers in the U.S. participated in the study.
[0132]The study was divided into Part A and Part B. The Part A was designed t...
example 2
A Randomized Double-Blind Placebo Control Study of TPX-100 in the Patients with Osteoarthritis of the Knees—Data Analysis with Stricter Qualification Standard Applied to the MRI Image Quality
[0225]Objectives
[0226]After the data analyses in EXAMPLE 1, a few more MRI images were questioned with regard to their technical quality to enable assessment of periarticular bone shape change over time. In order to assure the quality of the overall analysis results, a stricter qualification standard was applied to the MRI images used in EXAMPLE 1, and requalification was performed. Seventy-nine (79) subjects were requalified. The MRI knee images of these subjects were re-analyzed for their periarticular bone shape changes, as well as their correlations with the changes of tibiofemoral cartilage thickness and pain, respectively, in the same manner as described in EXAMPLE 1.
Results
[0227]The requalified 79 subjects were included in the efficacy analysis per the Statistical Analysis Plan.
[0228]Appr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Shape | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com