Agents for promoting tissue regeneration by recruiting bone marrow mesenchymal stem cells and/or pluripotent stem cells into blood
a technology of stem cells and blood, applied in the direction of drug compositions, peptide/protein ingredients, peptide sources, etc., can solve the problems of high treatment cost, poor cosmetic outcome, and qol in remarkably poor condition, so as to promote cell growth, promote functional regeneration/repair of damaged tissues, and promote cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
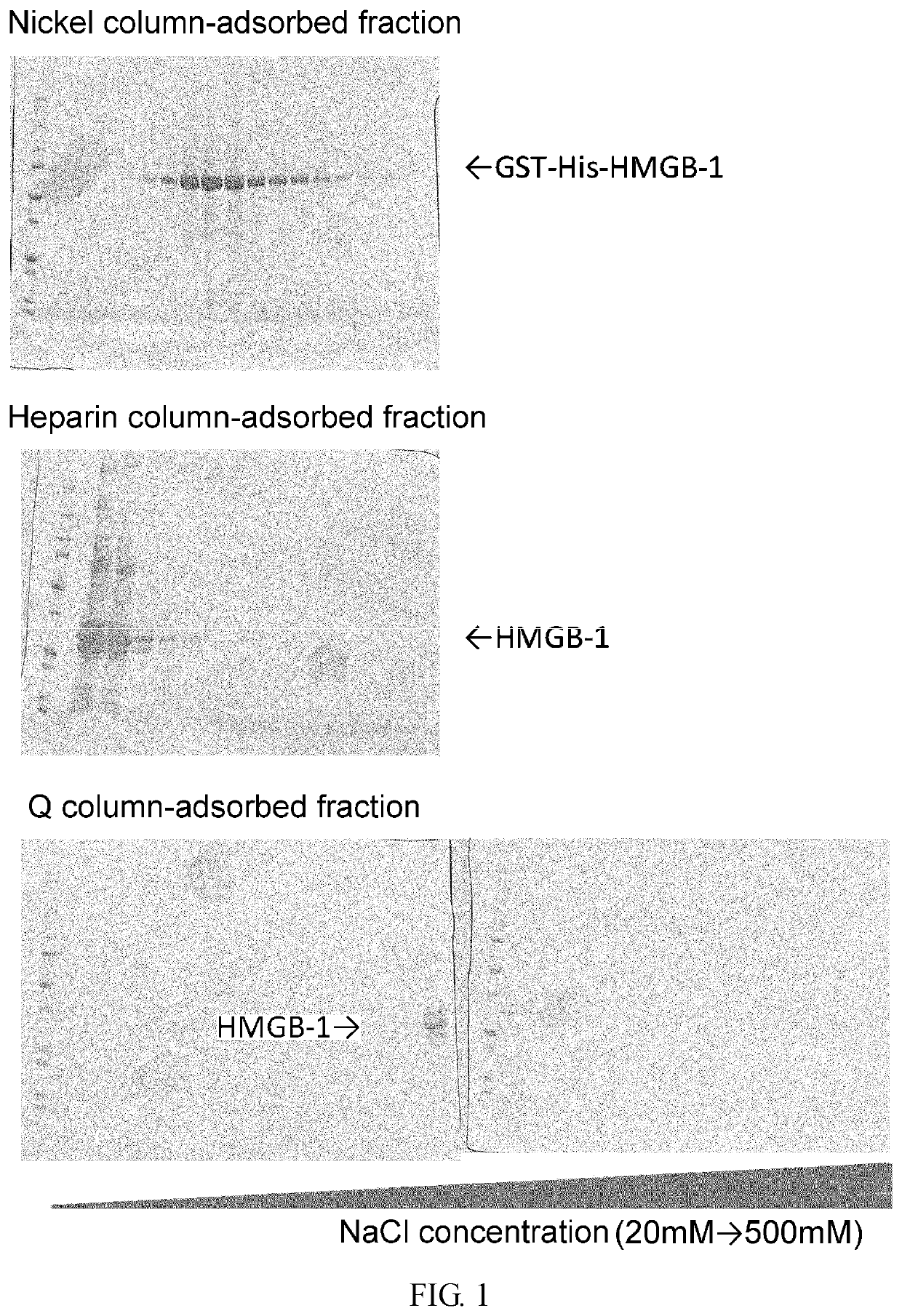
Purification of HMGB-1 and S100A8
[0350]RNA was extracted from newborn mouse skin using Trizol (Invitrogen), and then cDNA was synthesized using SuperScript III cDNA synthesis kit (Invitrogen). Using this cDNA as a template, HMGB1 cDNA was amplified by polymerase chain reaction (PCR). The resulting cDNA was inserted into pCAGGS, a plasmid vector for protein expression in mammalian cells, such that the vector would express the protein attached with GST tag and 6×His tag sequences at the N terminus of its amino acid sequence for the convenience of purification.
[0351]pCAGGS-Flag-His-S100A8 was transfected into a human fetal kidney cell-derived cultured cell line HEK 293 using polyethyleneimine (PEI). After 48 hours, the cells and culture supernatant were separately collected by centrifugation at 4,400 G at 4° C. for five minutes. Then, the collected supernatant was filtered through a cellulose acetate filter having pores with a diameter of 0.8 μm and then through a nitrocellulose filter...
example 2
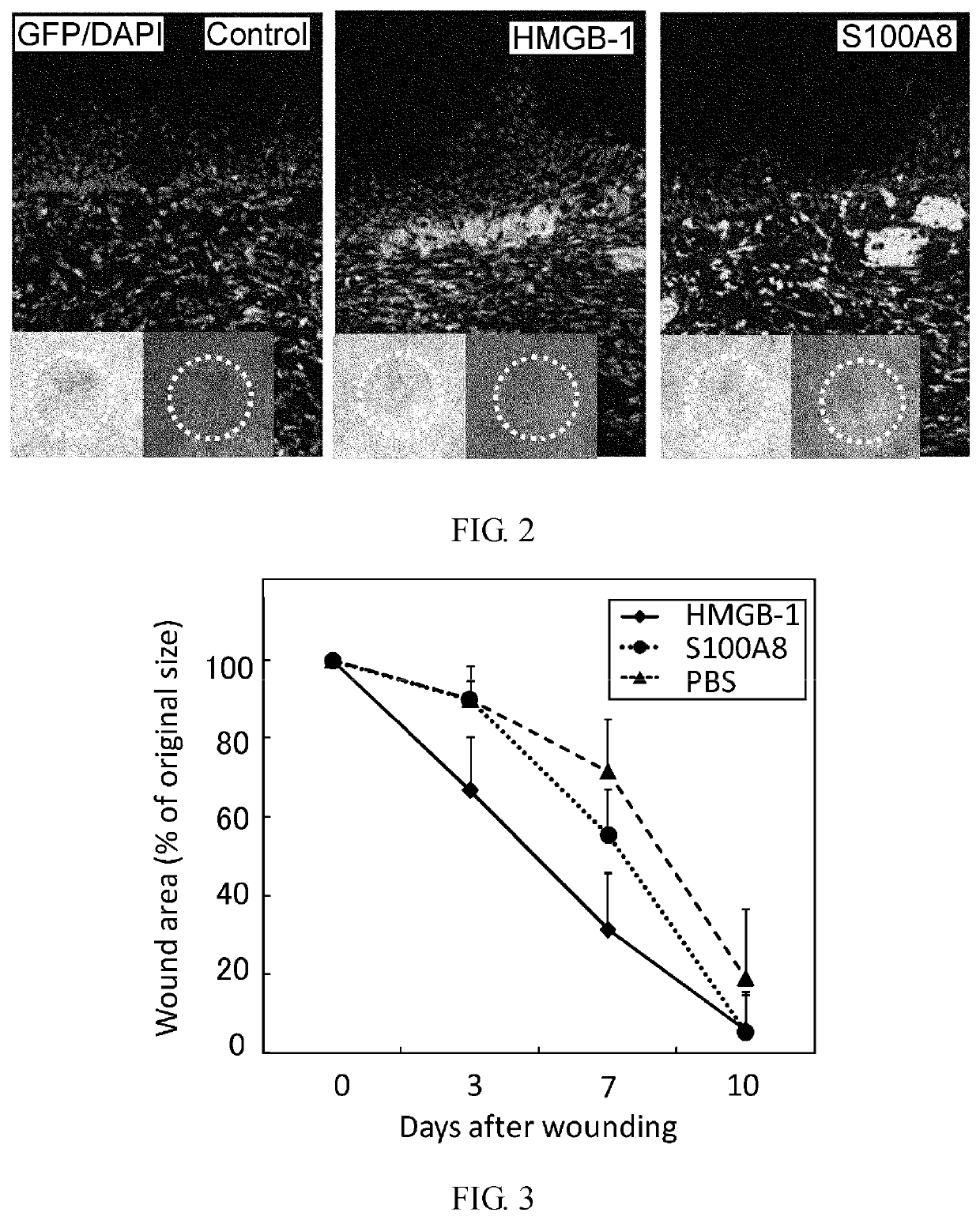
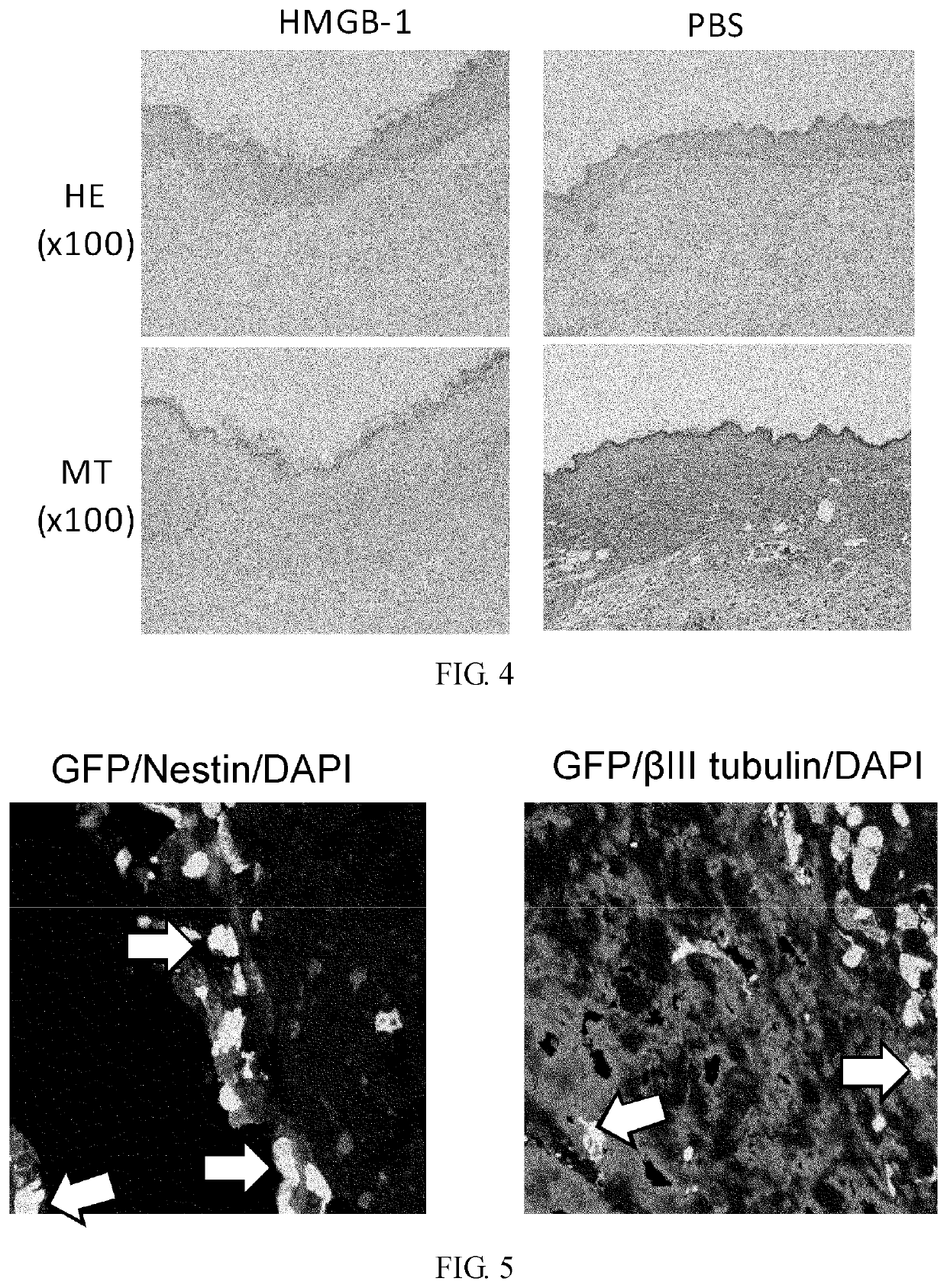
Effect of Intravenous Administration of HMGB-1 and S100A8 in Recruiting Bone Marrow-Derived Cells to Skin Ulceration Site During Skin Ulcer Healing Process
[0355]Male C57BL / 6 mice (6 weeks old) were irradiated at a lethal dose (10 Gy). Immediately, bone marrow cells (5×106 cells / 0.1 ml physiological phosphate buffer (pH 7.4)) derived from a green fluorescent protein (GFP) transgenic mouse (Okabe M. et al., FEBS Lett. 407, 313-319, 1997) were transplanted via the caudal vein. After 8 weeks, a round-shaped skin ulcer with a diameter of 6 mm was created on the back. To prevent shrinkage of the skin of the mice, a silicone ring with an outer diameter of 10 mm, inner diameter of 6 mm, and thickness of 1 mm was attached to the ulcer site using two-sided adhesive tape and medical adhesive Aron alpha A (Sankyo). The ulcer was covered with a silicone disc with a diameter of 10 mm and a thickness of 1 mm to prevent desiccation and bacterial infection at the ulcer. In addition, the ulcer was ma...
example 3
Effect of Intravenous Administration of HMGB-1 and S100A8 in Promoting Skin Ulcer Healing
[0359]In male C57BL / 6 mice (8 weeks old), a round-shaped skin ulcer with a diameter of 6 mm was created on the back. To prevent shrinkage of the skin of the mice, a silicone ring with an outer diameter of 10 mm, inner diameter of 6 mm, and thickness of 1 mm was attached to the ulcer site using two-sided adhesive tape and medical adhesive Aron alpha A (Sankyo). The ulcer was covered with a silicone disc with a diameter of 10 mm and a thickness of 1 mm to prevent desiccation and bacterial infection at the ulcer site. In addition, the ulcer was masked with Tegaderm (3M) for protection.
[0360]HMGB-1 (40 μg) or S100A8 (250 ng) was administered via the caudal vein five times at 24-hour intervals from the day of skin ulcer creation. The ulcer size was measured on days 3, 5, and 10 after creation of ulcer.
[0361]The result is shown in FIG. 3. HMGB-1 reduced the ulcer size on day 3 after creation of ulcer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com