Filament lamp for emission of yellow light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
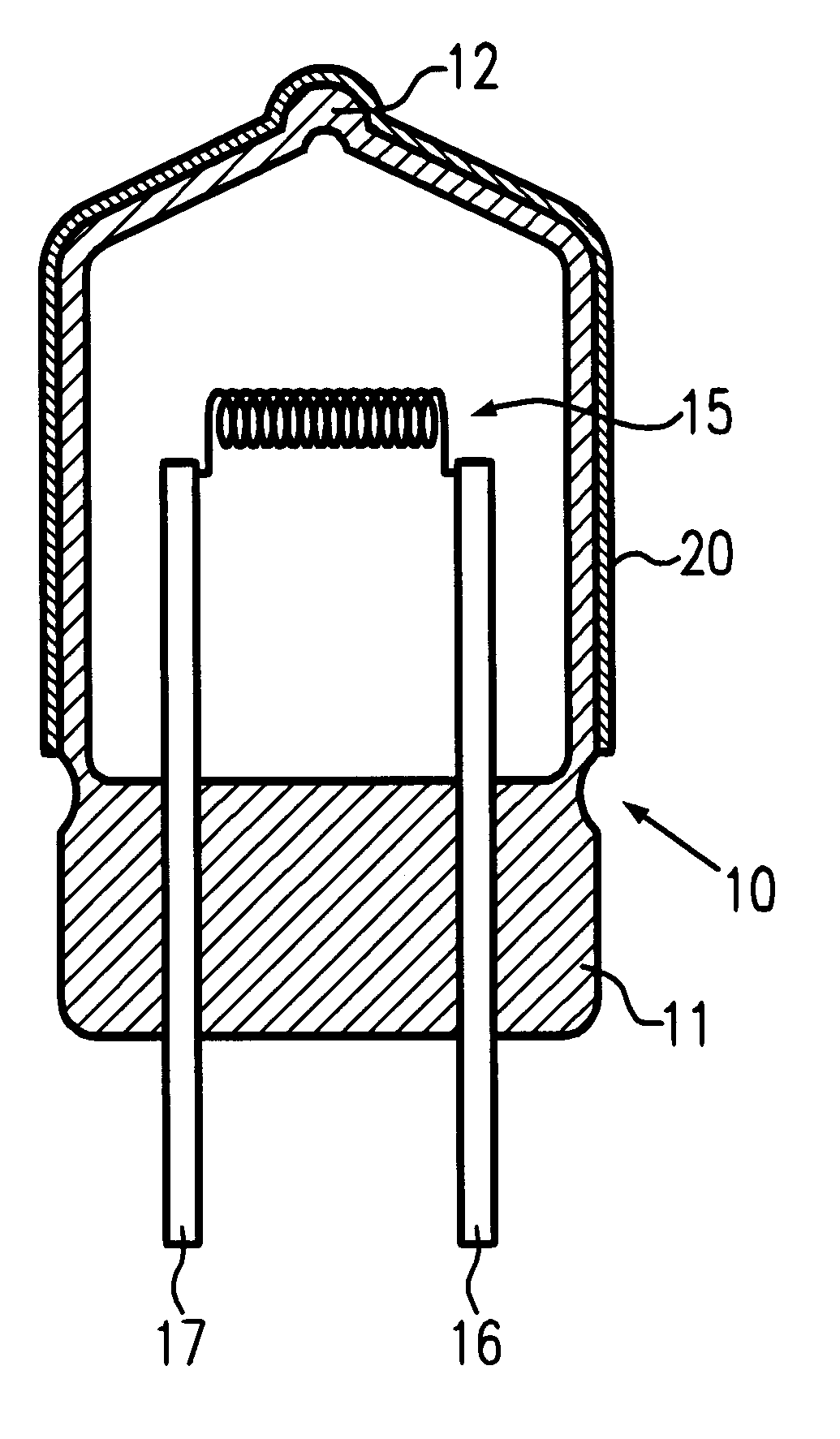
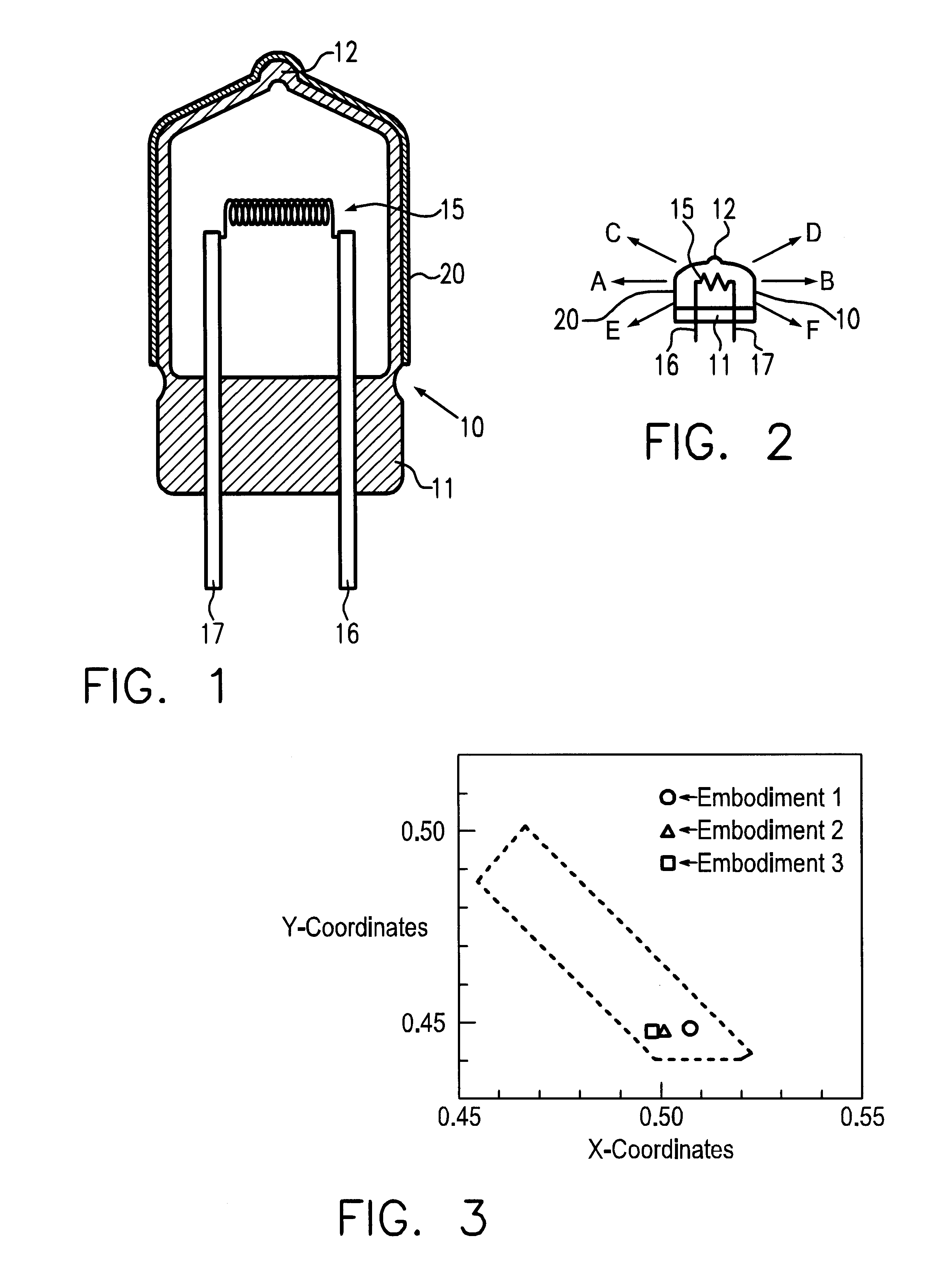
According to the arrangement shown in FIG. 1 a filament lamp with a rated output of 13.2 V and a power consumption of 55 W was produced in which color film is not formed. The chromaticity of the light emitted from this filament lamp has X-coordinates of 0.426 and Y-coordinates of 0.400.
A glass vessel was filled with a mixed organic solvent of ethanol and ethyl acetate (mixing ratio 50:50) with a percentage by weight of 69. Nickel nitrate-6-hydrate with a percentage by weight of 10 was added to this mixed organic solvent and stirred with a magnetic stirrer until the nickel nitrate was completely dissolved. Furthermore, antimony trioxide with a percentage by weight of 1 was added to this solution and stirred until the antimony oxide was completely dissolved. Afterwards titanium tetraisopropoxide with a percentage by weight of 12 was added to the resulting solution and reacted. The reaction mixture was then allowed to stand until the heat evolution by the reaction had decayed and the t...
embodiment 2
A glass vessel was filled with a mixed organic solvent of ethanol and ethyl acetate (mixing ratio 60:40) with a percentage by weight of 70. Nickel nitrate-6-hydrate with a percentage by weight of 8 was added to this mixed organic solvent and stirred with a magnetic stirrer until the nickel nitrate was completely dissolved. Furthermore, antimony trioxide with a percentage by weight of 0.8 was added to this solution and stirred until the antimony trioxide was completely dissolved. Afterwards titanium tetraisopropoxide with a percentage by weight of 12 was added to the resulting solution and reacted. This reaction mixture was then allowed to stand until the heat evolution by the reaction had decayed and the temperature of the reaction liquid had dropped to room temperature. Afterwards, the reaction liquid acetyl acetone with a percentage by weight of 9 was added and stirred for roughly 17 hours. In this way, the solution A was produced.
Furthermore, solution B was produced in the same w...
embodiment 3
In the production of the coating liquid in embodiment 2, the ratio of solution A to solution B was changed to a weight ratio of 80:20. Besides the production of the color film with a thickness of roughly 2.5 microns using this coating agent, a filament lamp in accordance with the invention for emission of yellow light was produced in the same way. In the color film, the ratio of titanium, nickel and antimony to one another was measured. The ratio by weight of titanium, nickel and antimony to one another was roughly 6.6:4:1. During operation of this filament lamp for emission of yellow light it was confirmed that yellow light is emitted.
In the above described filament lamp for emission of yellow light, cracking in the color film, transparency and heat resistance of the color film were evaluated in the same way as in embodiment 1. Furthermore, the chromaticity of the radiant light was measured. Table 1 shows the result.
As follows from the result in Table 1, in the filament lamps for e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com