Methylnaphthohydroquinone diphosphate sodium tablet and its preparing method
A technology for the yield of sodium naphthoquinone diphosphate tablet and sodium naphthoquinone diphosphate is applied in the field of sodium naphthoquinone diphosphate tablet and its preparation, and can solve the problems of drug lag and fluctuation, high fever, vomiting or Starvation, unsustainable thrombin levels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
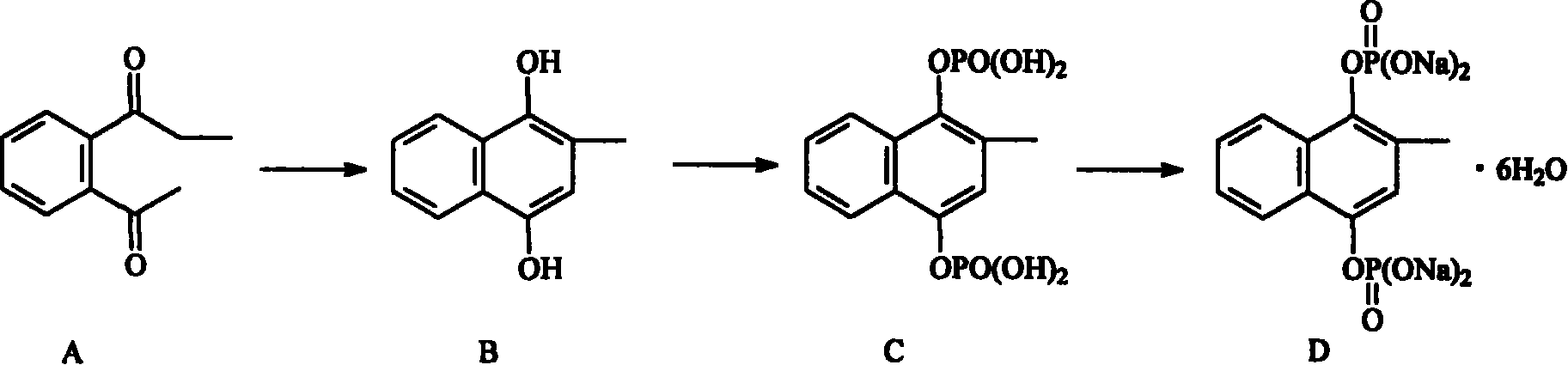
[0045] Using reactant A as a raw material, in accordance with the method suggested by Fessel·LF, Williamson·KL. Organic Experiment [M]. Beijing: Higher Education Press, 1986.370. The reduction reaction is carried out to obtain substance B;
[0046] Cool the mixture of dichloromethane and phosphorus oxychloride to -5°C, where the volume ratio of dichloromethane to phosphorus oxychloride is 10:3, while stirring, add B, triethylamine and dichloromethane dropwise at the same time The amount of the mixture is 0.1g / mL of B, 0.5g / mL of triethylamine, and 2 times the volume of dichloromethane; the temperature is controlled at -5℃~5℃, after the addition is complete, continue to stir for 10 Min, add 5 times the volume of pH 3 hydrochloric acid aqueous solution to wash, separate the layers, extract the aqueous layer with dichloromethane, discard the organic layer, extract the aqueous layer with isobutanol, combine the isobutanol layers, dry and concentrate, add petroleum Ether, th...
Embodiment 2
[0051]
[0052] Using reactant A as a raw material, in accordance with the method suggested by Fessel·LF, Williamson·KL. Organic Experiment [M]. Beijing: Higher Education Press, 1986.370. The reduction reaction is carried out to obtain substance B;
[0053] Cool the mixture of dichloromethane and phosphorus oxychloride to -5°C, where the volume ratio of dichloromethane to phosphorus oxychloride is 10:4, while stirring, add B, triethylamine and dichloromethane dropwise at the same time The amount of the mixture is 0.15g / mL of B, 0.4g / mL of triethylamine, and 2 times the volume of dichloromethane; the temperature is controlled at -5℃~5℃, after the addition is completed, continue to stir 15 Minutes, add 10 times the volume of pH 3 hydrochloric acid aqueous solution to wash, separate the layers, extract the aqueous layer with dichloromethane, discard the organic layer, extract the aqueous layer with isobutanol, combine the isobutanol layers, dry and concentrate, add petroleum Ether,...
Embodiment 3
[0058]
[0059] Using reactant A as a raw material, in accordance with the method suggested by Fessel·LF, Williamson·KL. Organic Experiment [M]. Beijing: Higher Education Press, 1986.370. The reduction reaction is carried out to obtain substance B;
[0060] Cool the mixture of dichloromethane and phosphorus oxychloride to -5°C, where the volume ratio of dichloromethane to phosphorus oxychloride is 10:5, while stirring, add B, triethylamine and dichloromethane dropwise at the same time The amount of the mixture is 0.2g / mL of B, 0.3g / mL of triethylamine, and 2 times the volume of dichloromethane; the temperature is controlled at -5℃~5℃, after the addition is complete, continue to stir for 20 Min, add 8 volumes of pH 3 hydrochloric acid aqueous solution to wash, separate the layers, extract the aqueous layer with dichloromethane, discard the organic layer, extract the aqueous layer with isobutanol, combine the isobutanol layers, dry and concentrate, add petroleum Ether, the solid i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com