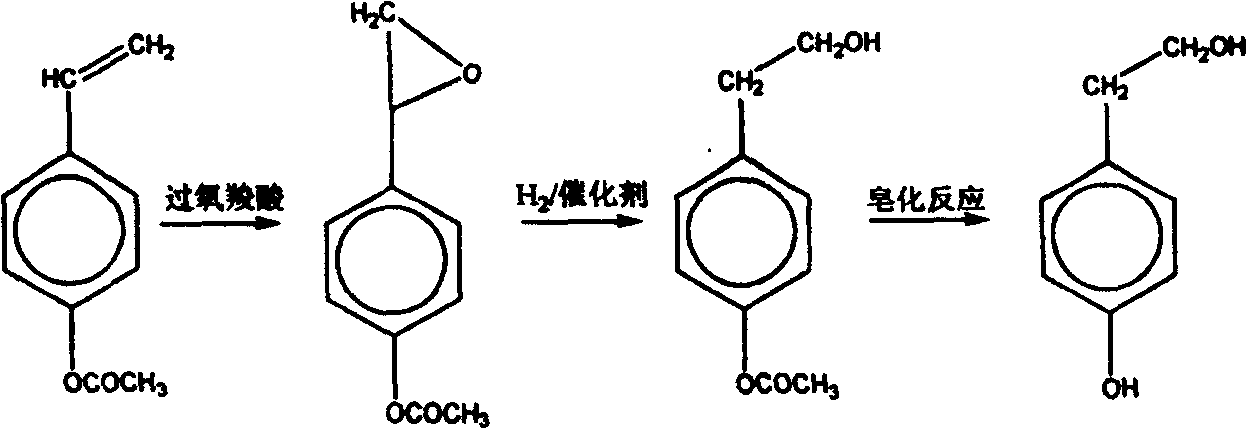
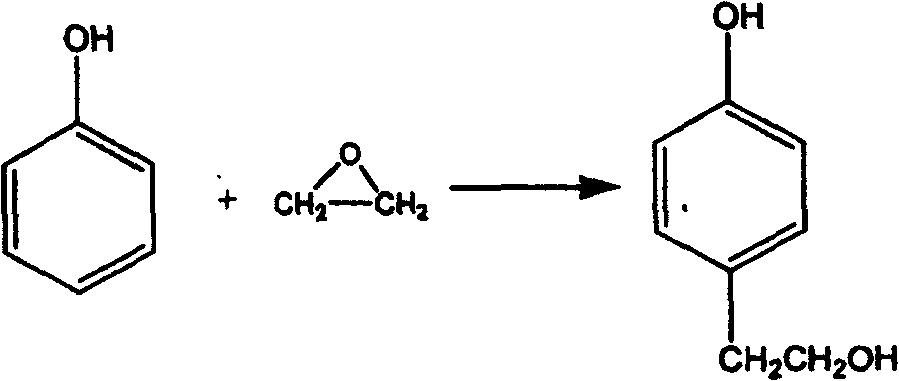
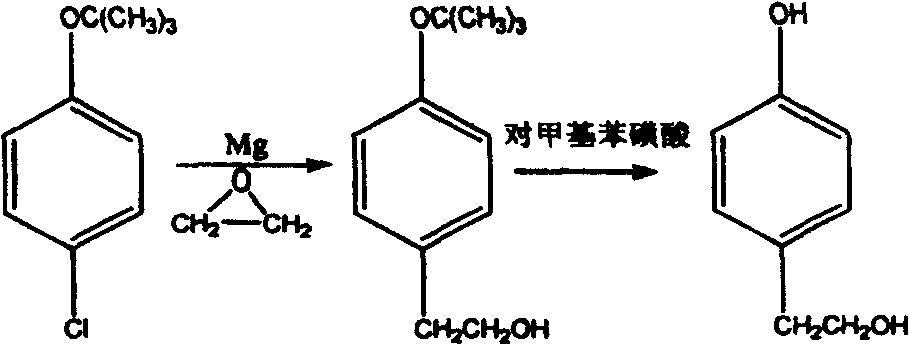
Technical method for synthesizing beta p-hydroxy phenethyl alcohol
A technique for the synthesis of p-hydroxyphenylethyl alcohol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of long synthetic process route, poor process practicability, low production cost, etc., and achieve the synthetic process route Short, low production cost, high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Esterification process: Take 47g (0.5mol) of phenol, 53mL (0.75mol) of acetic anhydride, add it to a 250mL three-necked flask with a reflux device, control the reaction temperature at 140±5°C, and react for 3.5 hours. The reaction is essentially complete. The obtained product is washed with water, then washed with sodium hydride, then washed with water, and then dried with anhydrous calcium chloride, the material after suction filtration is distilled, and the fraction at 190-200 ° C is collected to obtain phenyl acetate. The yield is was 90.8%.
[0035] (2) Alkylation process: get 13.6g (0.1mol) of phenyl acetate, the product of esterification process, 20mL (0.2mol) of 1,2-dichloroethane, and 0.5g phosphoric acid catalyst, add 70mLN , in a three-necked flask of N-dimethylformamide, keeping the reaction temperature at 100±5° C., a mixture of acetic acid-(4-β-haloethyl)phenyl esters was obtained after 6 hours. Then add 2 times of benzene to the mixture, wash with 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com