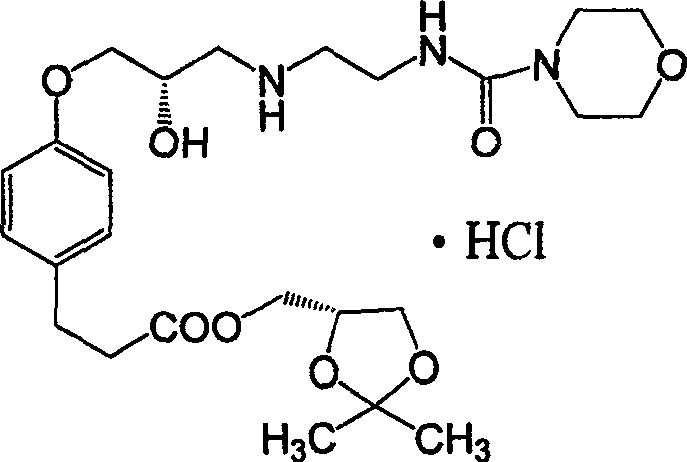
Method of synthesizing landiolol hydrochloride
A technique for the synthesis of landyrolol hydrochloride and a synthetic method, which is applied in the field of synthesis of landylol hydrochloride, can solve problems such as unfavorable industrial production, increase reaction cost, and reduce product yield, so as to be beneficial to industrial production and reduce synthesis cost , the effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Add 8.00ml S(+)-epichlorohydrin to 40.0ml acetone, slowly add 1.0ml boron trifluoride ether catalyst, and react at 40°C for 6 hours to obtain colorless and transparent (2,2 - Dimethyl-1,3-dioxolane-4S) methyl chloride liquid (product 1) 16.46 g, the yield is 95.5%.
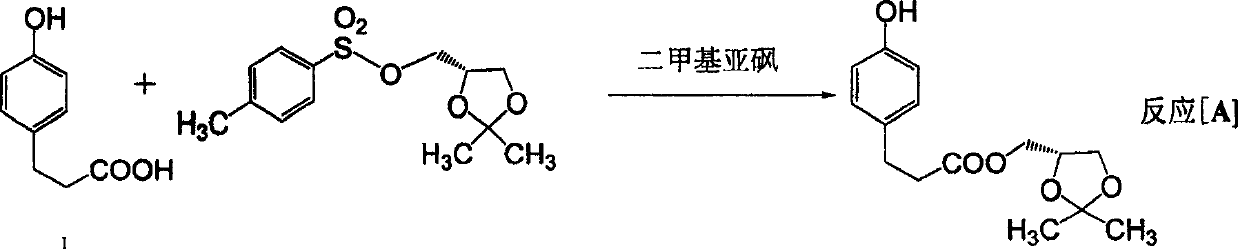
[0062] (2) Dissolve 8.30g of p-hydroxyphenylpropionic acid in 30.0ml of dimethyl sulfoxide, add 2.50g of potassium hydroxide and 8.20g of potassium carbonate, add 14.46g of product 1 dropwise into the reaction system, and react at 120°C 12 hours, be cooled to room temperature, filter, with the mixed solution of ethyl acetate and sherwood oil (V 乙酸乙酯 :V 石油醚 =1:5) extraction, washed with saturated sodium bicarbonate and sodium chloride, dried over magnesium sulfate and concentrated to separate 3-(4-hydroxyphenyl)propionic acid (2,2-dimethyl-1,3-di Oxolane-4S) methyl ester (product 2) 11.79g, its yield is 84.2%
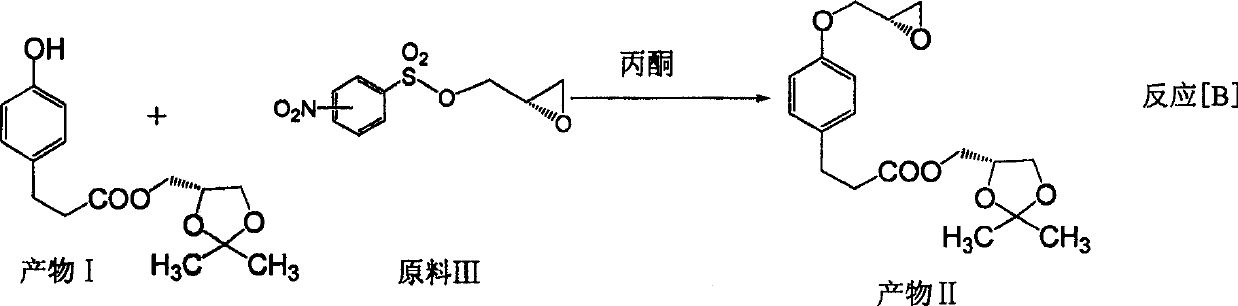
[0063] (3) Dissolve 5.6g of product 2 in 40.0ml of acetone, add 6.8g of anhydrous potassium...
Embodiment 2
[0068] In step (1), the reaction temperature was 45° C., and the weight of product 1 was 16.58 g, and the yield was 96.2%. Other steps are with embodiment 1.
Embodiment 3
[0070] In step (2), add 2.75g potassium hydroxide, with the mixed solution of ethyl acetate and sherwood oil (V 乙酸乙酯 :V 石油醚 =1:8) extraction, the weight of the precipitated product 2 was 12.35g, and the yield was 88.2%. Other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com