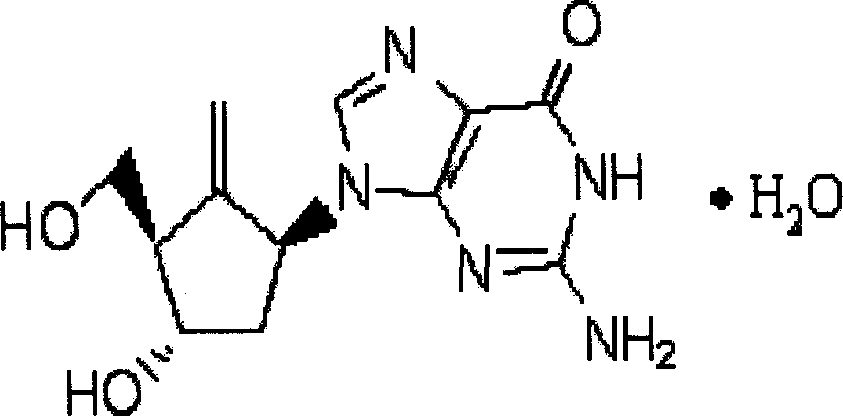
Method of preparing antivirotic entecavir hydrate
An antiviral drug, the technology of entecavir, applied in the field of compound preparation, can solve the problems of large-scale preparation difficulties, difficult reaction detection, low reaction yield, etc., and achieve simple product purification methods, high selectivity and stereoselectivity, and improved Effect of Reaction Conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
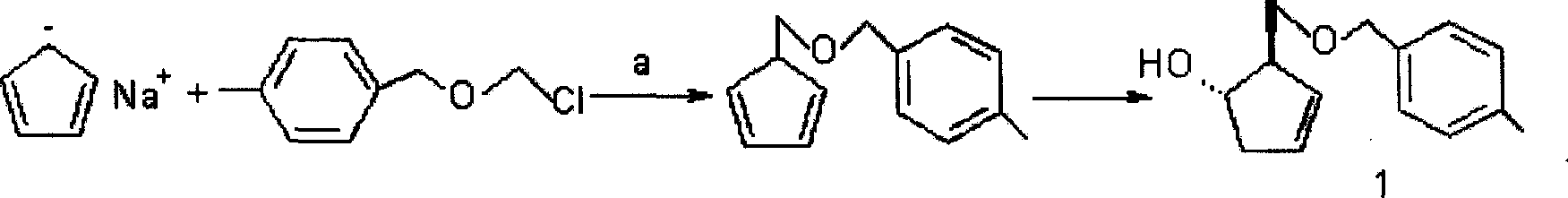
[0039] Example 1 (1S, 2R)-[(4-methylbenzyloxy)methyl]-3-cyclopenten-1-ol 1
[0040] Under anhydrous and oxygen-free conditions, anhydrous tetrahydrofuran (300.0 mL) and sodium hydride (32.2 g, 60%, 805.0 mmol) were mixed, stirred and cooled to below -10 ° C, and cyclopentadiene (80.2 mL, 882.2 mmol). After the dropwise addition was completed, the ice-salt bath was removed and left overnight at room temperature for later use.
[0041] Anhydrous tetrahydrofuran (300.0 mL) and p-methylbenzyl chloride methyl ether (150.8 g, 804.2 mmol) were mixed under anhydrous and oxygen-free conditions, cooled to -78 ° C, and then cyclopentadiene prepared in advance was added dropwise sodium tetrahydrofuran solution. After the dropwise addition was completed, the reaction was carried out at -78°C for 2 hours. stand-by.
[0042]Add anhydrous tetrahydrofuran (600.0mL) and cyclopentadiene sodium and chlorine The reaction mixture of methylbenzyl ether was stirred at -78°C for 2 hours after the...
Embodiment 2
[0043] Example 2. (S, 2R, 3S, 5R)-2-[(4-methylbenzyloxy)-methyl]-6-oxobicyclo[3.1.0]heptan-3-ol 2
[0044] Peroxy tert-butanol (65%, 80.0mL, 540.0mmol) mixed with 1,2-dichloroethane (120.0mL), at 0 ° C, N 2 Added dropwise to a mixture of cycloenol 1 (56.0g, 257.0mmol) and Vo(acac) under protection 2 (0.7g, 2.6mmol) in 1,2-dichloroethane (37.0mL), the dropwise addition was completed, and the temperature was controlled below 25°C for 16 hours. The reaction mixture was cooled to 0°C, and a saturated solution of sodium sulfite (280.0 mL) was added dropwise. After the addition was complete, stirring was continued at room temperature for 2 hours. Separate the organic phase, extract the aqueous phase with dichloromethane twice (200.0mL×2), combine the organic phases, wash with water, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 2 (57.0g) , which can be used in the next reaction without furt...
Embodiment 3
[0045] Example 3. (1S, 2R, 3S, 5R)-3-(benzyloxy)-2-[(4-methylbenzyloxy)-methyl]-6-oxobicyclo[3.1.0 ] Heptane 3
[0046] Epoxy alcohol 2 (50.5g, 215.8mmol) was dissolved in anhydrous tetrahydrofuran (160.0mL) under ice cooling, and added dropwise to a THF suspension containing sodium hydride (12.9g, 60%, 323.7mmol) under N2 protection . After the dropwise addition was completed, benzyl chloride (36.0 mL, 302.7 mmol) was added after the stirring was continued for 2 hours, and the mixture was stirred overnight under cooling in a water bath. The mixture was concentrated under reduced pressure, the residue was added with water (300.0 mL) and ethyl acetate (300.0 mL) and stirred well, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (150.0 mL×2), the organic phase was washed with water and saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Filter and concentrate to obtain 95.0 g of crude product. Column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com