Medical implantation material capable of by degraded by body fluid and its preparing process
A technology for implants and body fluids, applied in prosthetics, medical science, electrolytic coatings, etc., to achieve high support strength characteristics, good tissue compatibility and blood compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, preparation and performance test of medical Mg-Ca alloy implant
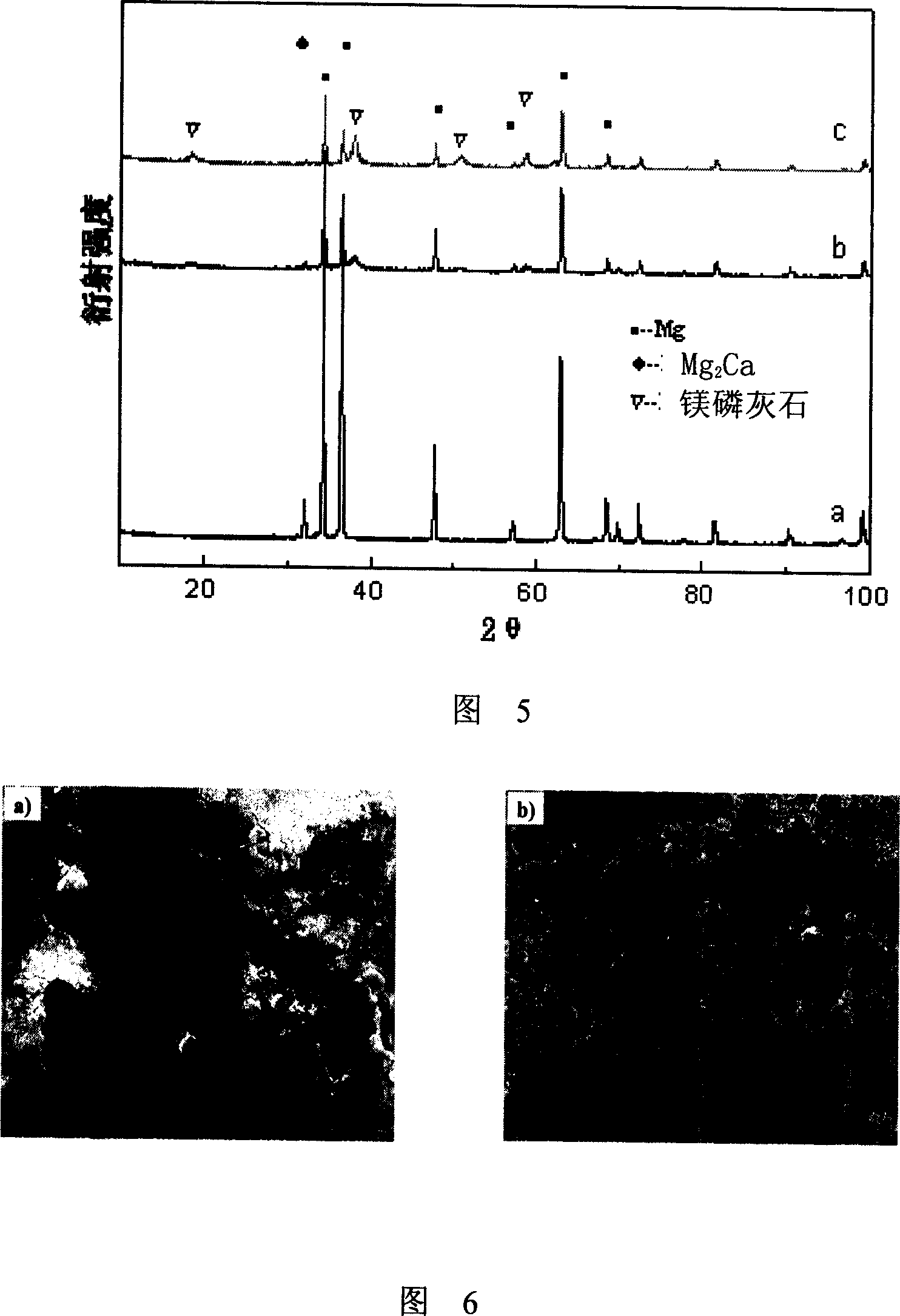
[0055] With pure Mg (purity is 99.9%), pure Ca (purity is 99.9%) (purchased from Beijing Cui Berlin Nonferrous Metals Technology Development Center) as raw materials, according to different mass ratios (magnesium calcium mass ratio is 99:1, 95:5 respectively) or 80:20) mixed in proportion, in CO 2 +SF 6 Under the protection of atmosphere, smelt at 700-720°C. After the raw materials are fully melted, after 10 minutes of heat preservation, the circulating water is rapidly cooled to obtain a Mg-Ca alloy ingot (Mg-1Ca (mass ratio of magnesium to calcium is 99:1), Mg- 5Ca (mass ratio of magnesium to calcium is 95:5), Mg-20Ca (mass ratio of magnesium to calcium is 80:20)) (Figure 1), the phase composition is detected by X-ray diffraction (XRD), and some results are shown in Figure 2, The results show that the second phase Mg does not appear when the mass content of calcium increases to 1% (Mg-1C...
Embodiment 2
[0065] Example 2. Preparation of biodegradable medical implant and its cytotoxicity test
[0066] Using pure Mg (purity 99.9%) and pure Ca (purity 99.9%) (purchased from Beijing Cuibin Nonferrous Metals Technology Development Center) as raw materials, the mass ratio of magnesium to calcium is 99:1, mixed in CO2+SF6 atmosphere Under protection, smelt at 700-720°C. After the raw materials are fully melted, keep warm for 10 minutes, and then cool quickly with circulating water to prepare Mg-Ca alloy ingot Mg-1Ca (mass ratio of magnesium to calcium is 99:1).
[0067] Sterilize and sterilize 5 magnesium-calcium alloy blocks prepared above with a length, width, and thickness of 10, 10, and 1 mm respectively, and place them in a sterile culture bottle. The volume ratio of 1640 culture solution is 3cm 2 Add RPMI 1640 culture solution at a ratio of / ml, place in 37°C, 95% relative humidity, and 5% CO2 incubator for 72 hours to obtain the original solution of magnesium-calcium alloy ex...
Embodiment 3
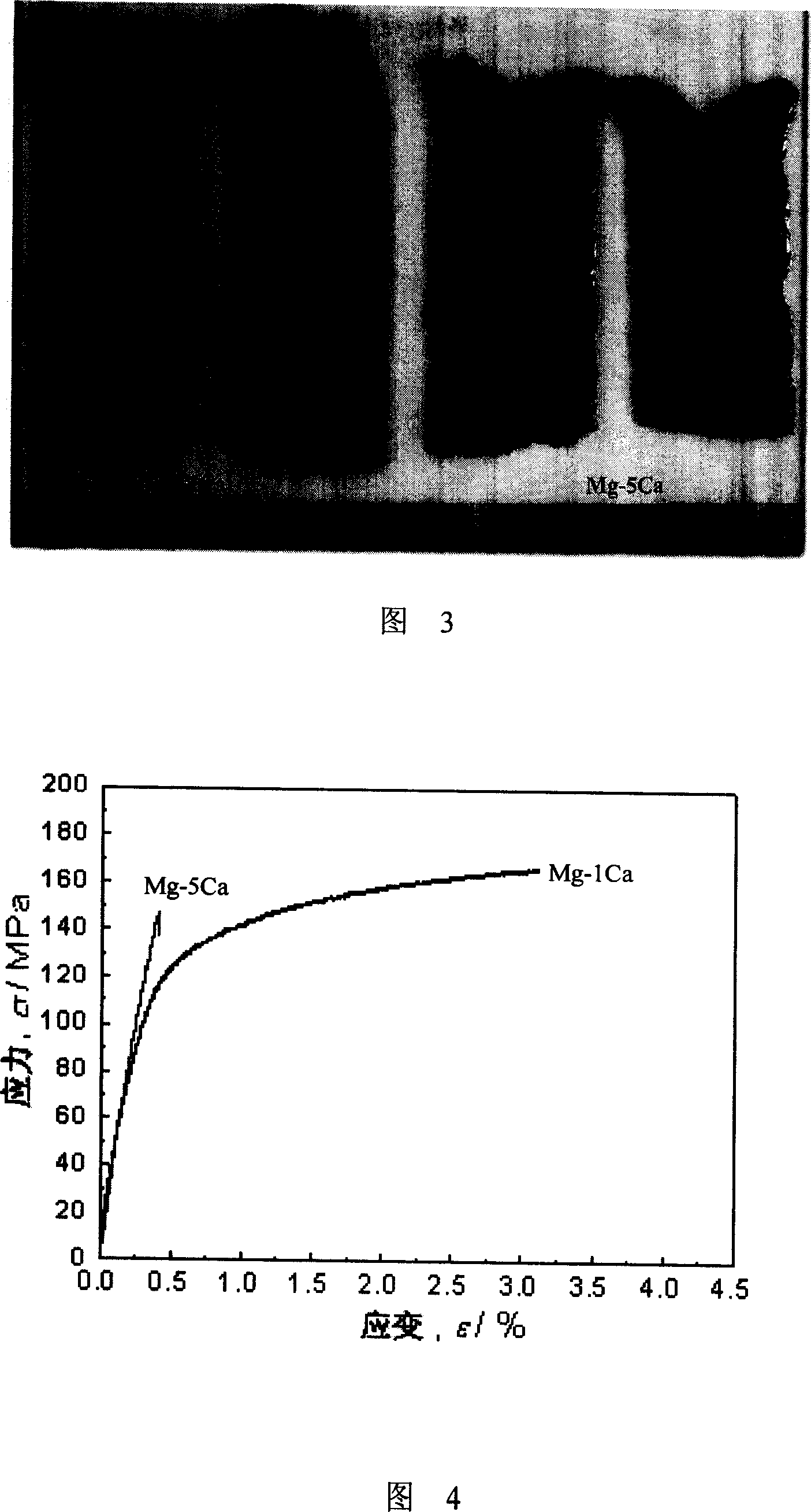
[0069] Example 3. Preparation of biodegradable medical implant and its mechanical properties test
[0070] The rapid solidification Mg-Ca alloy (Mg-2Ca (the mass percent composition of magnesium is 98 ± 0.3%, the mass percent composition of calcium is 2 ± 0.3%), Mg-5Ca (magnesium mass percent composition) is prepared by high vacuum rapid quenching system. The mass percent composition is 98±0.3%, the mass percent composition of calcium is 5±0.3%), Mg-5Ca-5Zn (the mass percent composition of magnesium is 98±0.3%, the mass percent composition of calcium is 5±0.3%) 0.3%, the mass percentage of Zn is 5 ± 0.3%)) thin strip, the specific method is: after mixing the raw materials according to the stated ratio, use a high vacuum rapid quenching system to prepare rapid solidification Mg-2Ca, Mg-5Ca or Mg- 5Ca-5Zn thin strip, the parameters are feed amount 2~8g, induction heating power 3-7kW, distance between nozzle and roller 0.80mm, injection pressure 0.1MPa, roller speed 2000r / mln and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thread length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com