Double function catalyst for synthesizing polycarbonate
A bifunctional catalyst and polycarbonate technology, applied in the field of catalysts, can solve the problems of long reaction time, high pressure, low catalyst activity, etc., and achieve the effects of high polymer product selectivity, mild reaction conditions and high catalyst activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
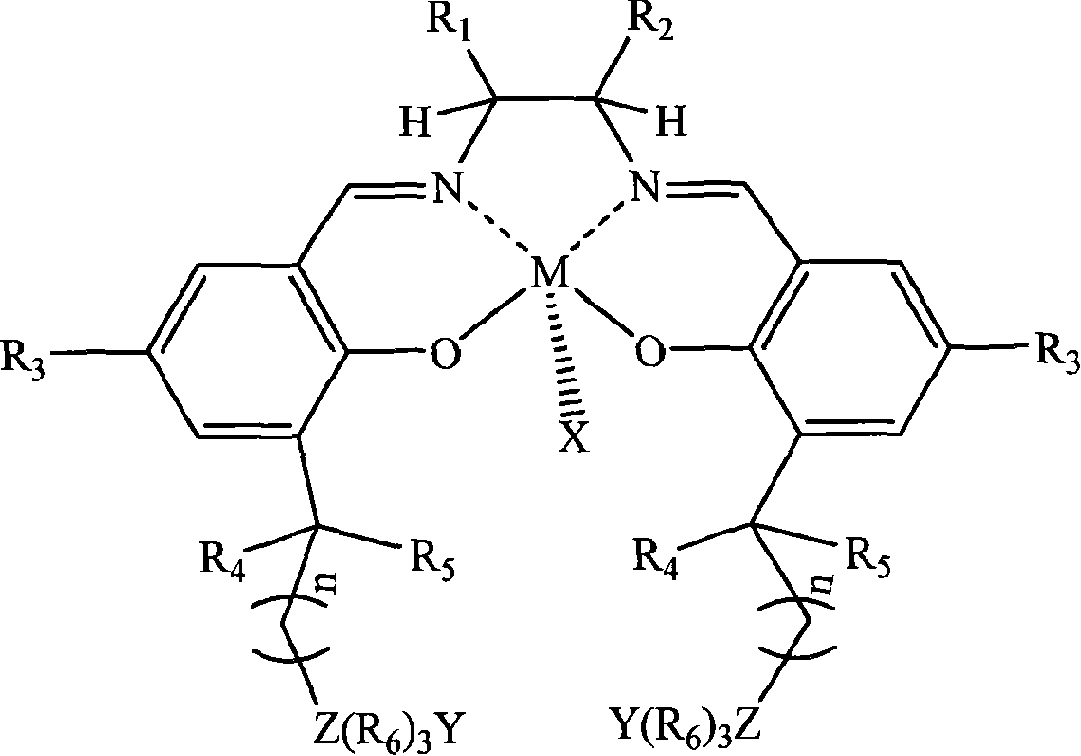
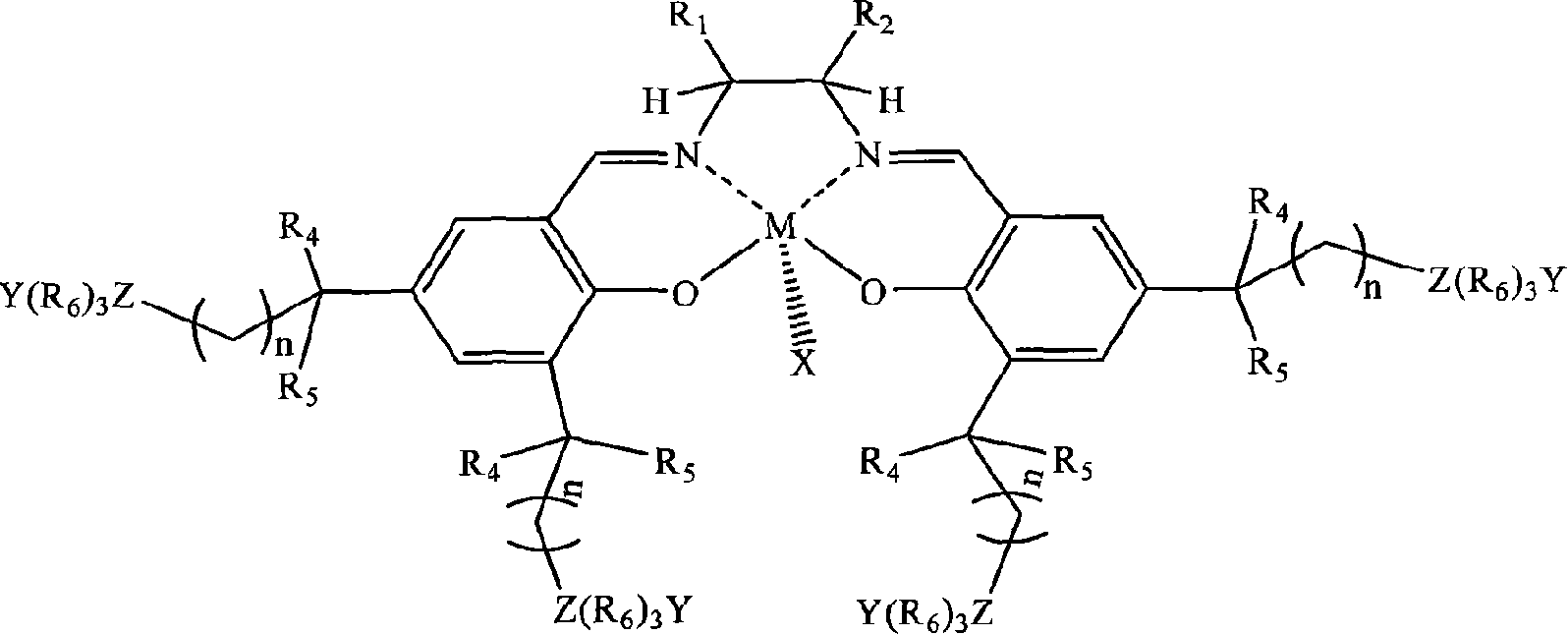
[0026] In a stainless steel autoclave with an effective volume of 200ml, add in the following order at ambient temperature: 0.1 mmol of tetradentate Schiff base cobalt complex (X, Y are 2,4-dinitrophenol oxyanions; R 1 = R 2 = H; R 3 is tert-butyl; R 4 , R 5 is methyl; R 6 n is 2; Z is nitrogen; the quaternary ammonium salt group is at the 5th position of the benzene ring) and 1 mole of propylene oxide, and then carbon dioxide gas is introduced to maintain a constant pressure of 2.0 MPa. Control the temperature at 50°C, react under magnetic stirring for 6 hours, slowly release unreacted carbon dioxide in the autoclave, collect unreacted propylene oxide in a cold trap at -20°C, and then add a certain amount of methanol / chloroform mixture The polymer was dissolved, and then a large amount of ether was added to precipitate polycarbonate. It was filtered, washed several times with ether, and dried in vacuo to constant weight to obtain 37 g of polypropylene carbonate as a whit...
Embodiment 2
[0028] In the same equipment used in Example 1, under the same conditions, only the diamine skeleton in the tetradentate Schiff base cobalt complex was changed from ethylenediamine (i.e. R 1 = R 2 =H) is replaced by cyclohexanediamine (i.e. R 1 , R 2 for -(CH 2 ) 4 -)). After reacting at 50° C. for 6 hours, 39 g of polypropylene carbonate was obtained with a molecular weight of 62,000 and a molecular weight distribution of 1.33, and the carbonate units in the polymer exceeded 99%.
Embodiment 3
[0030] In the same equipment used in Example 1, under the same conditions, only the diamine skeleton in the tetradentate Schiff base cobalt complex was changed from ethylenediamine (i.e. R 1 = R 2=H) is replaced by o-phenylenediamine (i.e. R 1 , R 2 for-(CH) 4 -)). After reacting at 50° C. for 6 hours, 38 g of polypropylene carbonate was obtained, the molecular weight was 59400, the molecular weight distribution was 1.29, and the carbonate units in the polymer exceeded 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com