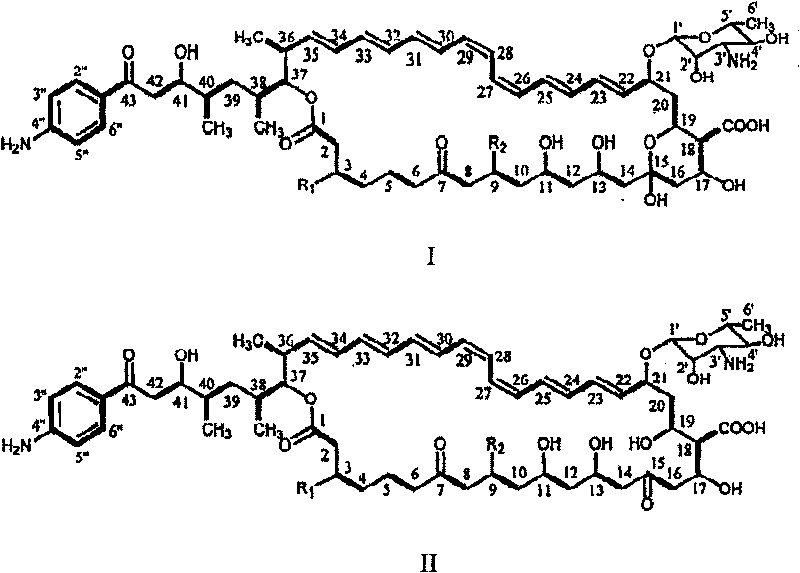
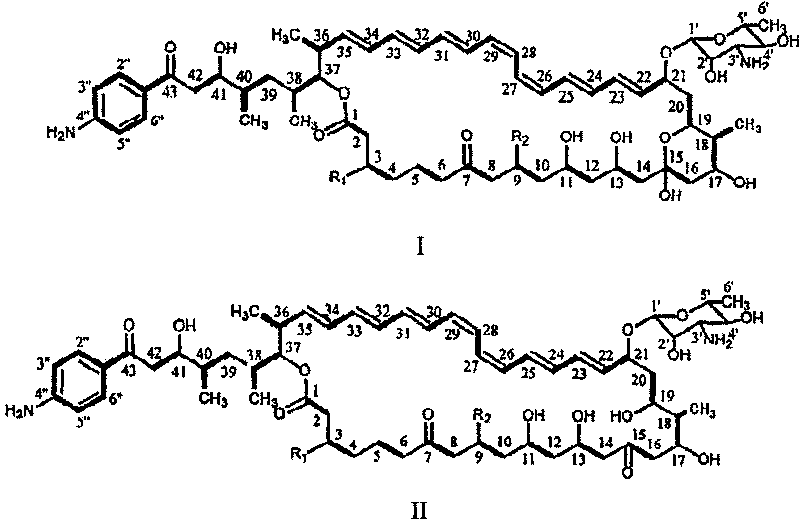
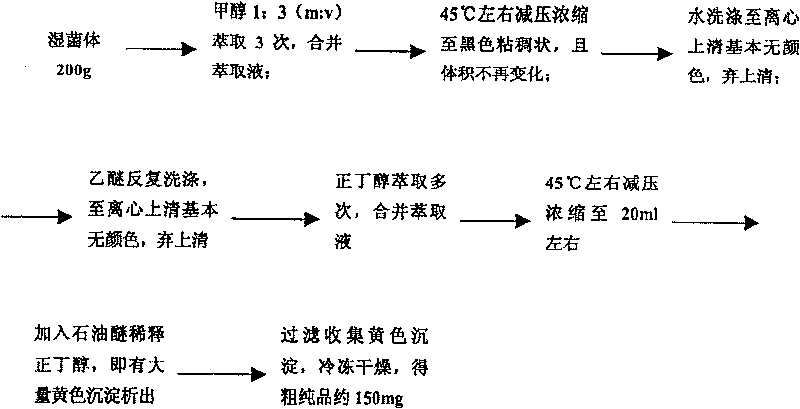
Decarboxylated FR-008 derivative polyketone antibiotic and use thereof
A technology of FR-008 and antibiotics, applied in the field of biomedicine, can solve problems such as high cost, unbearable for patients, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Construction of gene replacement plasmid for cytochrome P450 monooxygenase gene fscP
[0045] Based on the sequence information obtained from the FR-008 gene cluster, a gene replacement plasmid for the cytochrome P450 monooxygenase gene fscP was constructed. pJTU28 is a derivative clone of pIJ2925 carrying the 2.3 kb Pst I-Kpn I fragment containing the fscP gene. pJTU28 was obtained by self-ligating pJTU26 after digestion with PstI. pJTU26 is a clone in which the 6.6 kb Kpn I fragment from pHZ145 was inserted at the pIJ2925 Kpn I cloning site. There are two BssH II restriction sites 165 and 561 bases downstream of the start codon of fscP in the cytochrome P450 monooxygenase gene. After pJTU28BssH II digestion, the 396bp sequence inside the fscP gene was deleted, and the 1.4kb fragment containing the apramycin resistance gene excised from pJTU33 was inserted to obtain pJTU37. pJTU33 is a derivative plasmid from the BamH I fragment containing the apramycin r...
Embodiment 2
[0046] Example 2: Screening of deletion mutants of fscP gene
[0047] The gene replacement plasmid pJTU50 was introduced into Streptomyces FR-008 by conjugative transfer of two parents, and after two rounds of relaxation culture without antibiotics, a mutant strain CS103 resistant to apramycin and sensitive to thiostrepton was obtained by counter selection .
Embodiment 3
[0048] Example 3: Fermentation of the deletion mutant strain CS103 of the fscP gene
[0049] Medium (m / v): glucose 2%, starch 1%, glycerol 1.389%, yeast powder 0.3%, industrial amino acid powder 0.5%, peptone 0.101%, NaCl 1%, FeSO 4 ·7H 2 O 0.05%, CuSO 4 0.00428%. Before sterilization, the pH was adjusted to 6.90 with 5 mol / l NaOH solution, the inoculation time was 30 hours, the inoculum size (v / v) was 10%, and the culture was carried out at 28 degrees and 200 rpm for 4 days on a shaking table.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com