Porous gel polyelectrolyte thin film and preparation method thereof
A technology of gel polymer and electrolyte film, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of insufficient liquid electrolyte adsorption, complex solvent extraction steps, low porosity, etc., and achieve convenient product separation, Strong polarity and good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
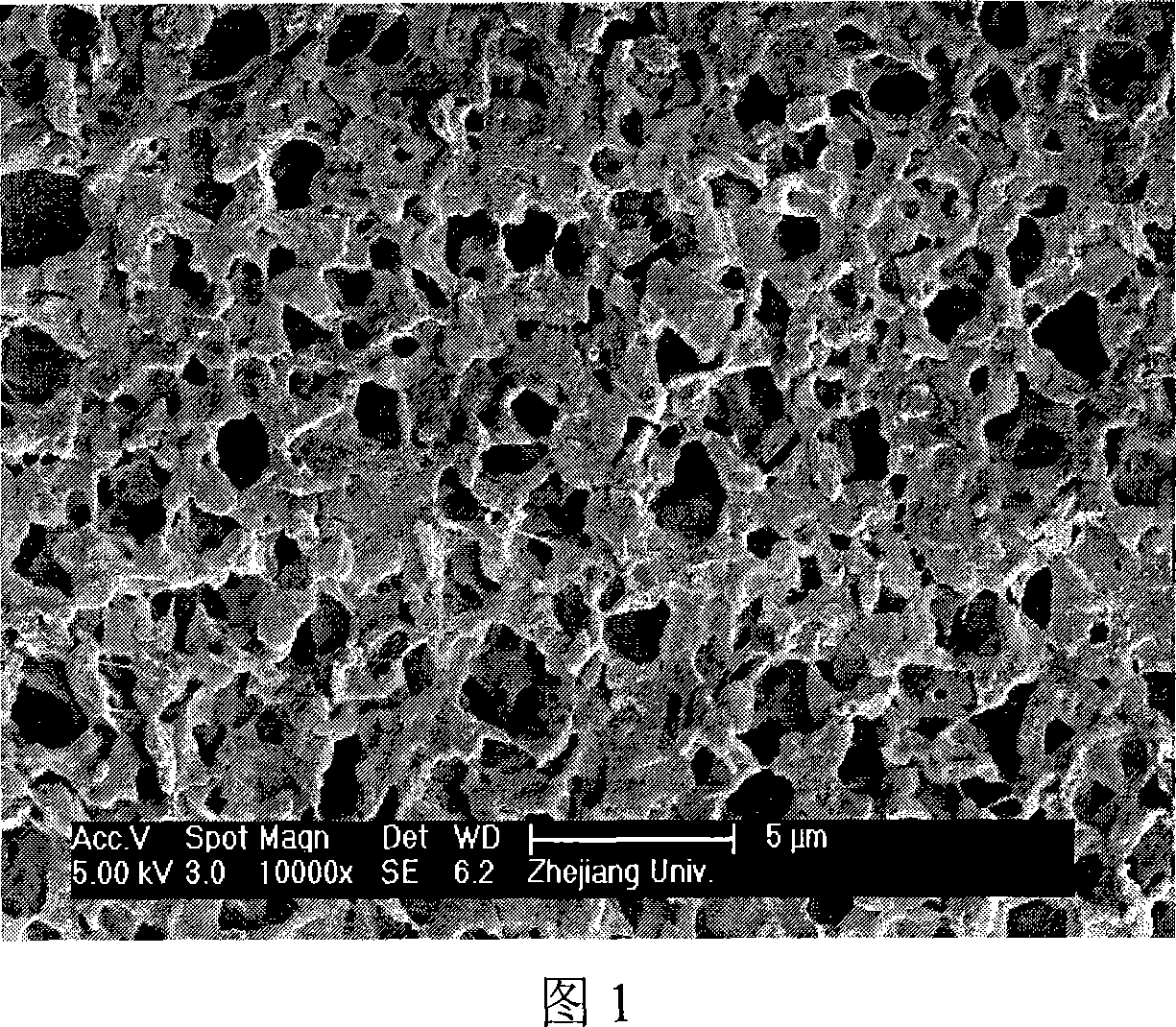
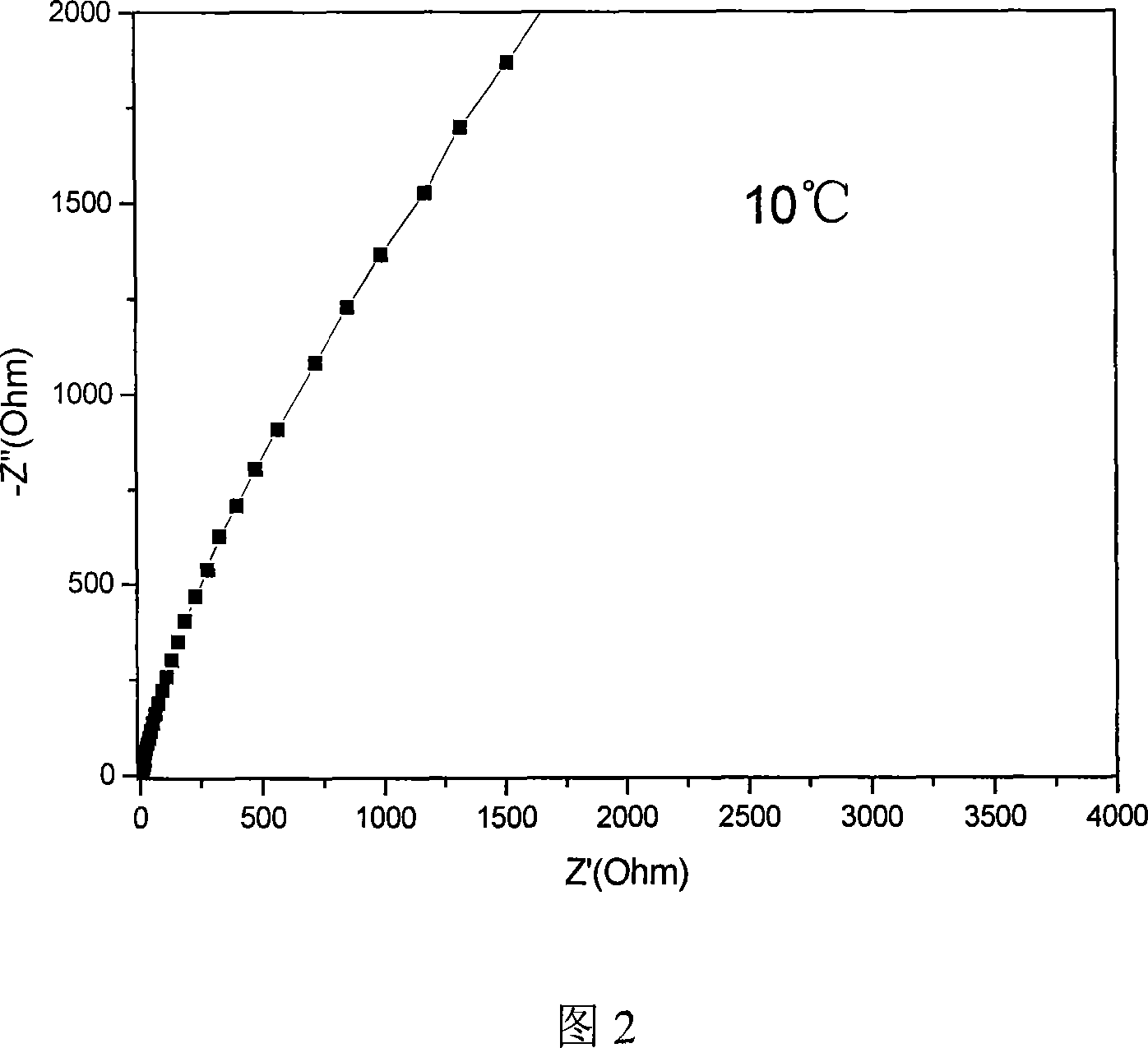
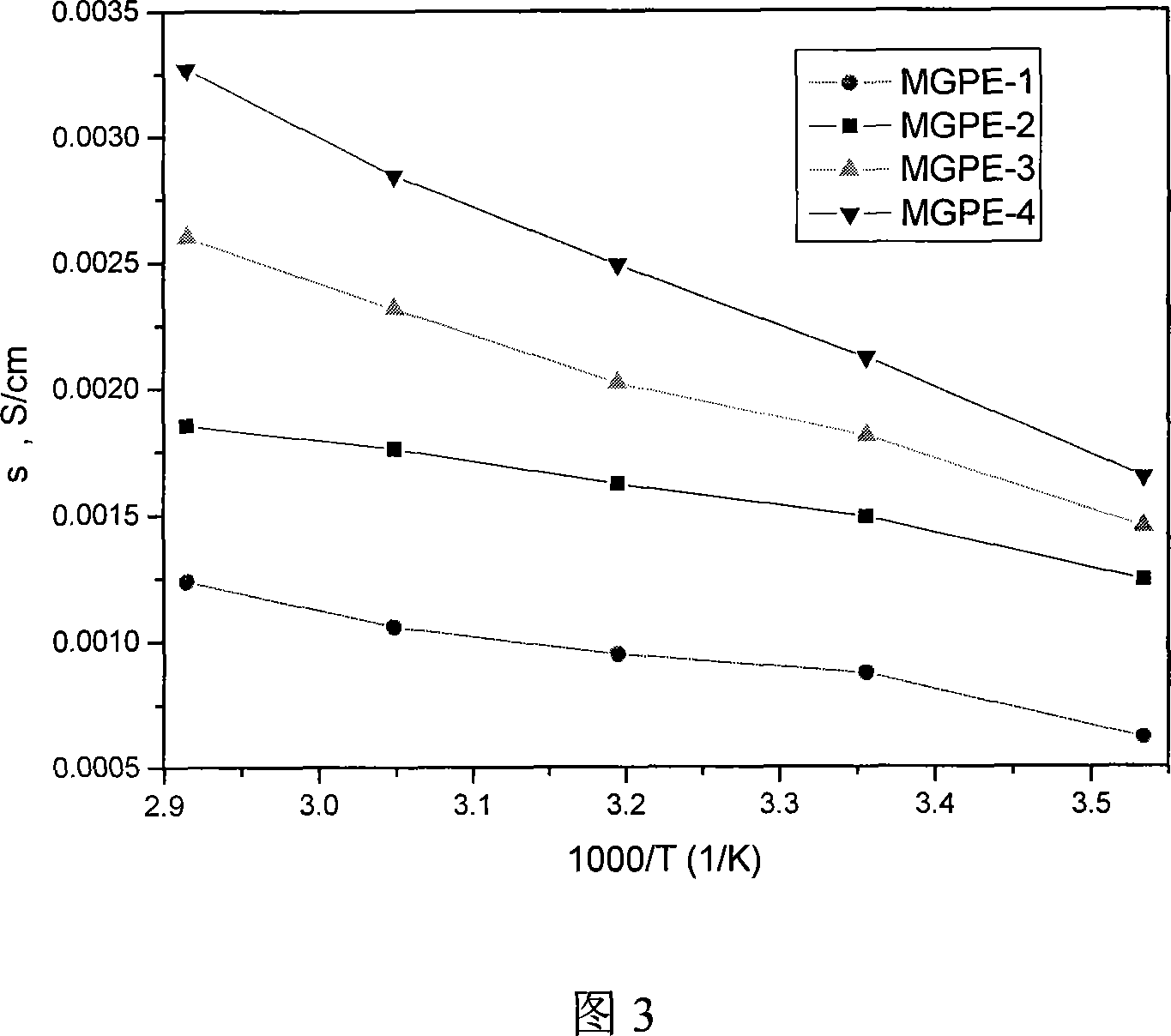
Image
Examples
Embodiment 1
[0017] 1) In a 100ml three-necked flask, 2.42g of polyethylene glycol monomethyl ether methacrylate and 5.3g of acrylonitrile were blended and dissolved in 26ml of ethanol, and stirred evenly. After adding 0.054 g of azobisisobutyronitrile, nitrogen gas was introduced for 30 minutes, then the temperature was raised to 70° C., and the mixture was reacted for 8 hours to obtain a transparent viscous liquid. Then pour it into a 100ml single-necked flask, evaporate the ethanol solvent by a rotary evaporator, and dry at 50°C for 24 hours under vacuum to obtain a light yellow waxy solid, which is an acrylonitrile-polyethylene glycol monomethyl ether methacrylate copolymer;
[0018] 2) Get 2.1g polyvinylidene fluoride and 0.9g polyethylene glycol monomethyl ether ester-acrylonitrile copolymer blend and dissolve in 20g N, N-dimethylacetamide (DMAC) solvent, at 50 ℃ magnetic stirring for 48 hours, dissolved evenly. After the polymer blend was cooled to room temperature, the polymer sol...
Embodiment 2
[0023] 1) In a 100ml three-necked flask, 2.42g of polyethylene glycol monomethyl ether methacrylate and 2.65g of acrylonitrile were blended and dissolved in 16.9ml of ethanol, and stirred evenly. After adding 0.035 g of azobisisobutyronitrile, argon was introduced for 30 minutes, then the temperature was raised to 80° C., and the mixture was reacted for 8 hours to obtain a transparent viscous liquid. Then pour it into a 100ml one-necked flask, and evaporate the ethanol solvent by a rotary evaporator. Drying under vacuum at 50°C for 24 hours yielded a pale yellow waxy acrylonitrile-polyethylene glycol monomethyl ether methacrylate copolymer;
[0024] 2) 2.7 g of polyvinylidene fluoride and 0.3 g of polyethylene glycol monomethyl ether methacrylate-acrylonitrile copolymer were blended and dissolved in 15 g of DMAC solvent, stirred magnetically at 50° C. for 48 hours, and dissolved uniformly. After the blended polymer was cooled to room temperature, the polymer solution was coat...
Embodiment 3
[0026] 1) with embodiment 1 step 1)
[0027]2) 2.4 g of polyvinylidene fluoride and 0.6 g of polyethylene glycol monomethyl ether methacrylate-acrylonitrile copolymer were blended and dissolved in 17 g of DMAC solvent, stirred magnetically at 50° C. for 48 hours, and dissolved uniformly. After the blended polymer was cooled to room temperature, the polymer solution was coated on a glass plate with a stainless steel scraper, and then immersed in deionized water to obtain a porous film. After immersing the obtained porous film in n-hexane for 24 h, first Vacuum-dry at room temperature for 6 hours, then vacuum-dry at 60°C for 24 hours. Obtain a porous membrane with a thickness of 100 μm, which is immersed in a 1M lithium hexafluorophosphate carbonate electrolyte solution. The carbonate electrolyte solution of 1M lithium hexafluorophosphate is composed of dimethyl carbonate, diethyl carbonate and ethylene carbonate, According to the mass ratio of dimethyl carbonate: diethyl carbo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com