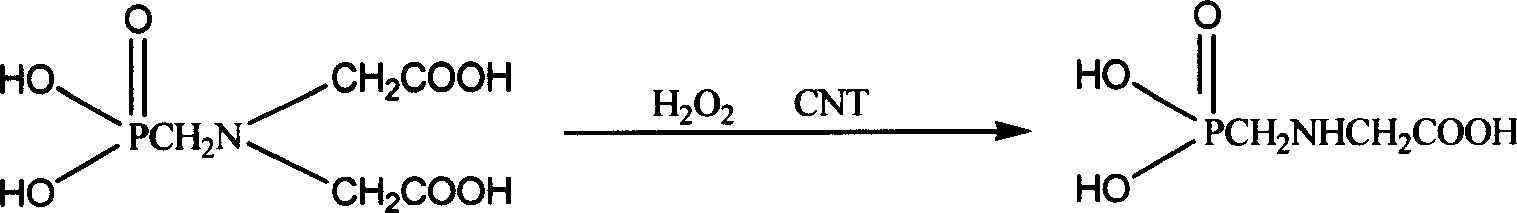Method for preparing glyphosate by catalytic oxidation method
A technology for catalytic oxidation and glyphosate, which is applied in chemical instruments and methods, organic chemistry, compounds of Group 5/15 elements of the periodic table, etc., can solve the problems of easy deactivation of catalysts, expensive precious metals, and difficulty in recycling, etc. Achieve good economic, social and environmental benefits, improve color and quality, and reduce material consumption and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Mix 5 grams of carbon nanotubes (typical multi-walled carbon nanotubes prepared by Fe-Mo / Al2O3) with about 100 milliliters of concentrated nitric acid, heat to 90 degrees Celsius, and keep stirring for 3 to 4 hours. After filtering, the carbon nanotubes on the filter residue were washed with deionized water until neutral.
[0043] Add 5 grams of carbon nanotubes obtained by the above method, 100 milliliters of water, 20 grams of PMIDA, and 20 grams of hydrogen peroxide in a 250 milliliter three-necked flask, heat and stir, and wait for the temperature to rise to 75 ° C, analyze and detect that the diglycophosphine disappears, about 3 -5 hours, in situ selectivity 92%.
Embodiment 2
[0045] Carbon nanotubes were recovered in Example 1, 100 milliliters of water was added, and then ammonia water was added to adjust the pH to 9, stirred at room temperature for 30 minutes, filtered, and the filter residue was washed with 40 milliliters of deionized water; 100 milliliters of water was added to the filtered carbon nanotubes , adding dilute hydrochloric acid to adjust the pH to 2, stirring at room temperature for 30 minutes, filtering, washing the filter residue with deionized water until neutral, and setting aside.
[0046] Mix the recovered carbon nanotubes in Example 1 treated above with 100 milliliters of water, 20 grams of PMIDA, and 20 grams of hydrogen peroxide, heat to 65° C., and analyze and detect that the bisglyphosate disappears. It takes about 3-5 hours. The selectivity is 92.7%.
Embodiment 3
[0048] Carbon nanotubes were recovered in Example 2, 100 milliliters of water was added, and then ammonia water was added to adjust the pH to 9, stirred at room temperature for 30 minutes, filtered, and the filter residue was washed with 40 milliliters of deionized water; 100 milliliters of water was added to the filtered carbon nanotubes , adding dilute hydrochloric acid to adjust the pH to 2, stirring at room temperature for 30 minutes, filtering, washing the filter residue with deionized water until neutral, and setting aside.
[0049] Mix the recovered carbon nanotubes in Example 2 above with 100 milliliters of water, 20 grams of PMIDA, and 20 grams of hydrogen peroxide, heat to 70° C., and analyze and detect that the bisglyphosate disappears. It takes about 3-5 hours. Selectivity 90.06%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com