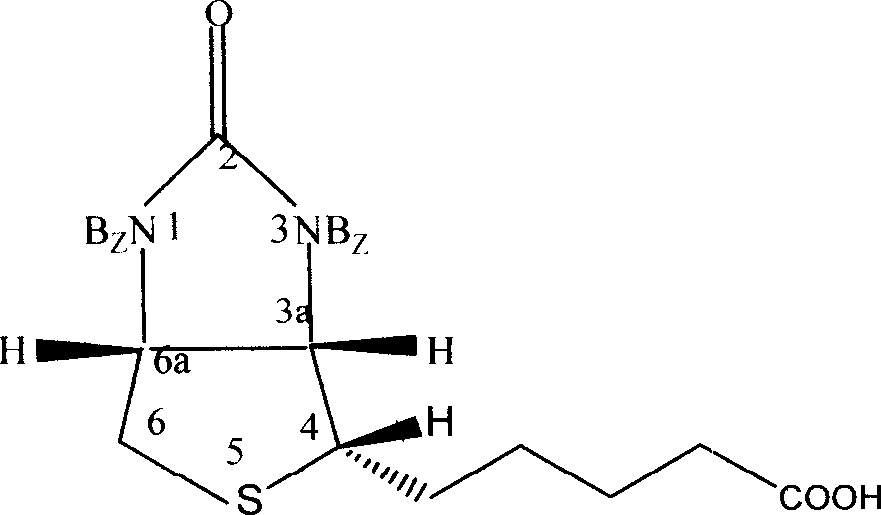
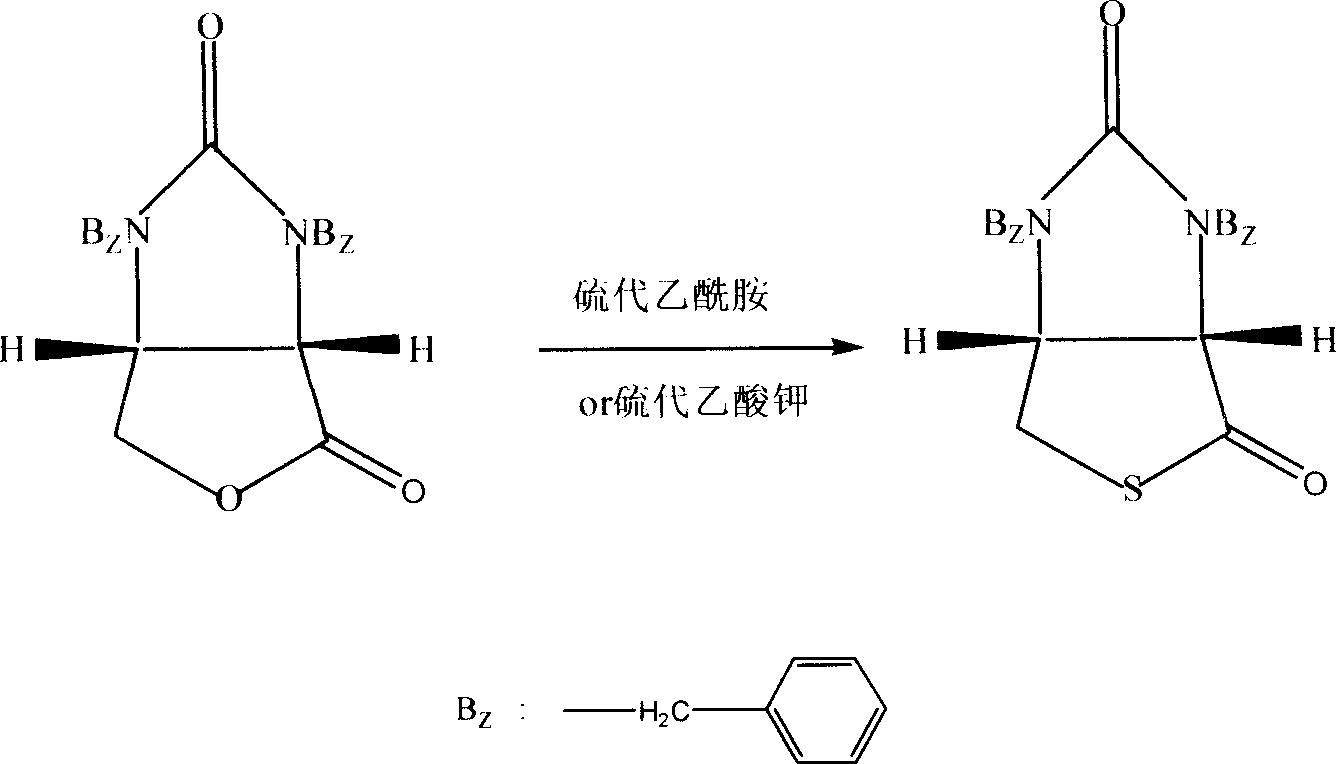
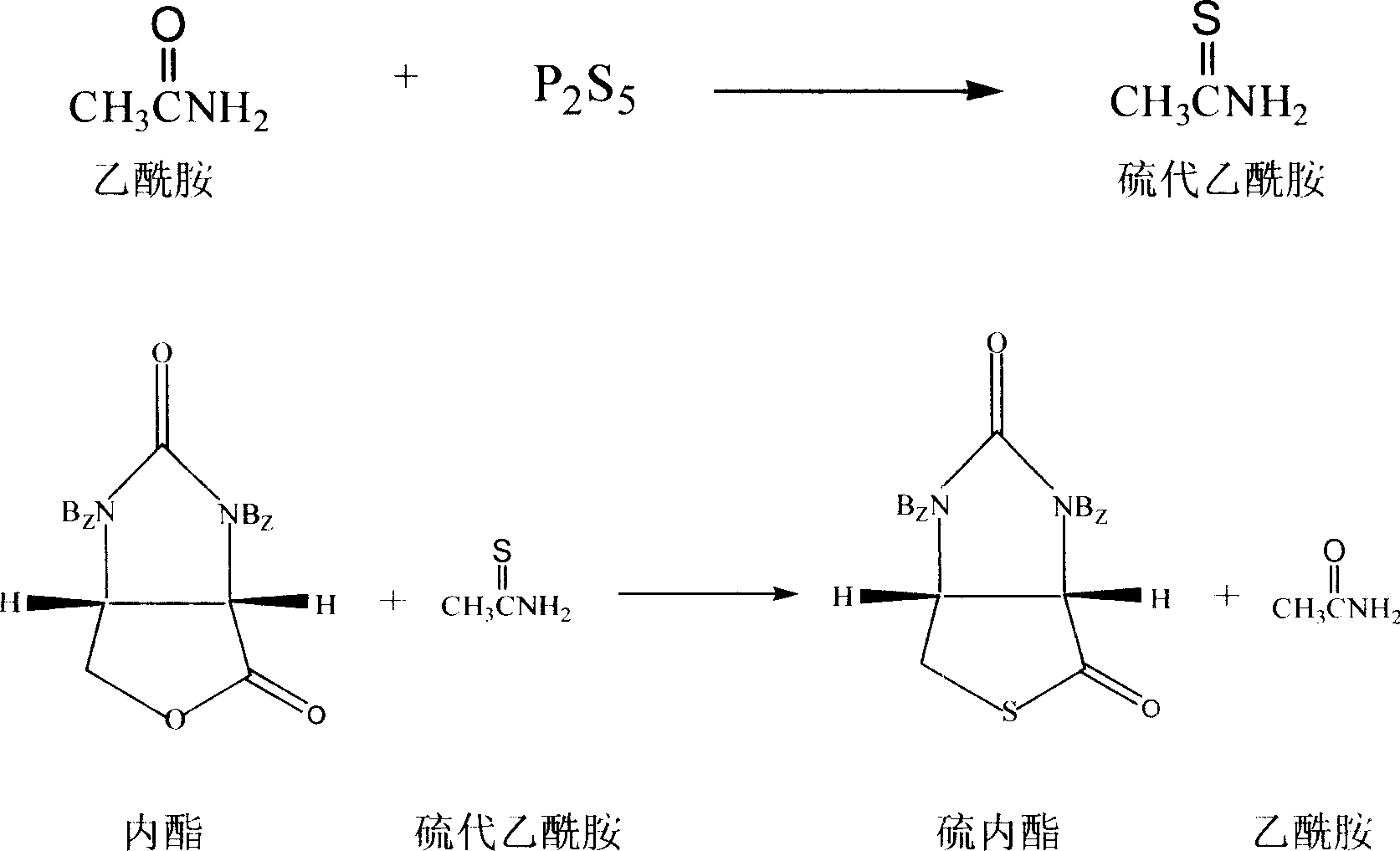
Process for synthesizing thiolactone
A synthesis process, thiolation technology, applied in the direction of organic chemistry, can solve the problems of high unit consumption, large environmental pollution, low yield, etc., and achieve the effect of increasing reaction yield, reducing cost, and easily obtaining raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 500ml three-necked flask with a reflux condenser, a stirrer, and a thermometer, add 20.0g (0.062mol) of raw material lactone, 10g (0.045mol) of phosphorus pentasulfide, 2.6g (0.044mol) of acetamide, N,N-dimethyl 150ml of formamide solution was heated in an oil bath to control the external temperature to 150°C, kept at reflux for 2 hours, and the reaction was complete by HPLC detection. Control the external temperature below 100°C, recover N,N-dimethylformamide under reduced pressure to obtain a dark brown solid, add 200ml of toluene while it is hot, stir to form a uniform suspension, add 5% hydrochloric acid dropwise, and adjust the pH to PH =2, separate the organic layer, then extract the aqueous layer with 50ml of toluene, combine the organic layers, wash the organic layer with saturated aqueous sodium bicarbonate solution to neutrality, then wash it once with deionized water, recover the toluene under reduced pressure to obtain a slightly pink color Solid, soake...
Embodiment 2
[0033] In a 500ml three-neck flask equipped with a co-current condenser, a stirrer, and a thermometer, add 20.0g (0.062mol) of raw material lactone, 10g (0.045mol) of phosphorus pentasulfide, 0.3g (0.005mol) of acetamide, N,N-dimethyl 150ml of methyl formamide solution, heated in an oil bath to control the external temperature of 150°C, and kept at reflux for 2 hours. The HPLC detection reaction was carried out by about 70%, and the HPLC detection reaction was basically complete after continuing to react for 3 hours. The aftertreatment method is the same as in Example 1 to obtain a white solid, which is 19.5g after vacuum drying, with a melting point of 125-127°C and an optical rotation [α] D 25 =+91.8 0 (c1.0, CHCl 3 ), the HPLC content is 98.7%, and the yield is 93.2%.
Embodiment 3
[0035] Others are the same as in Example 2, the amount of acetamide charged is 3 g (0.05 mol), and the reflux reaction is carried out for 1.5 h to obtain 19.0 g of the product with a yield of 90.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com