Eu(III)-Fe(II) luminous nano-tube and its preparation method and use
A technology of nanotubes and luminescent materials, applied in the application field of biomolecular recognition, can solve the problems of scarcity of three-dimensional structures, difficulty in selecting ligands, and difficulty in predicting and controlling products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of the present invention comprises the following steps:
[0025] 1) Eu 2 o 3 , FeSO 4 ·7H 2 O and PDA are mixed in a molar ratio of 1:3~4:5.5~6, put into the polytetrafluoroethylene liner of the hydrothermal reaction kettle, and first add 4-6mL CH 3 CN, followed by the rapid addition of 7-9 mL H 2 O, without stirring, in case Fe 2+ It is oxidized and sealed tightly with a stainless steel sleeve.
[0026] 2) Rapidly raise the temperature to a high temperature of 145-155° C., and keep the temperature constant for 70-72 hours.
[0027] 3) Cool down to room temperature at a rate of 0.5-1° C. / hour, filter, and wash twice with deionized water to obtain dark red polyhedral prism crystals suitable for single crystal diffraction.
Embodiment 1
[0028] Embodiment 1 [Eu(PDA) 3 Fe 1.5 (H 2 O) 3 ]·1.5H 2 Synthesis of O:
[0029] 0.2mmol Eu 2 o 3 (0.070g), 0.6mmol FeSO 4 ·7H 2 O (0.167g), 1.2mmol PDA (0.200g) mixture, put into the polytetrafluoroethylene liner of 20mL hydrothermal reaction kettle, add 4mLCH first 3 CN, followed by the rapid addition of 8mL H 2 O, without stirring, in case Fe 2+ After being oxidized and sealed tightly with a stainless steel sleeve, the temperature was quickly raised to a high temperature of 150°C, and after a constant temperature of 72 hours, the temperature was programmed to cool down to room temperature. The obtained product was washed twice with 5mL water, and finally a deep red polyhedral prism suitable for single crystal diffraction was selected. shaped crystals. The calculated yield based on metal Eu was 65%.
Embodiment 2
[0030] Embodiment 2 [Eu(PDA) 3 Fe 1. 5(H 2 O) 3 ]·1.5H 2 Characterization of O:
[0031] (1) [Eu(PDA) 3 Fe 1.5 (H 2 O) 3 ]·1.5H 2 Structure determination of O
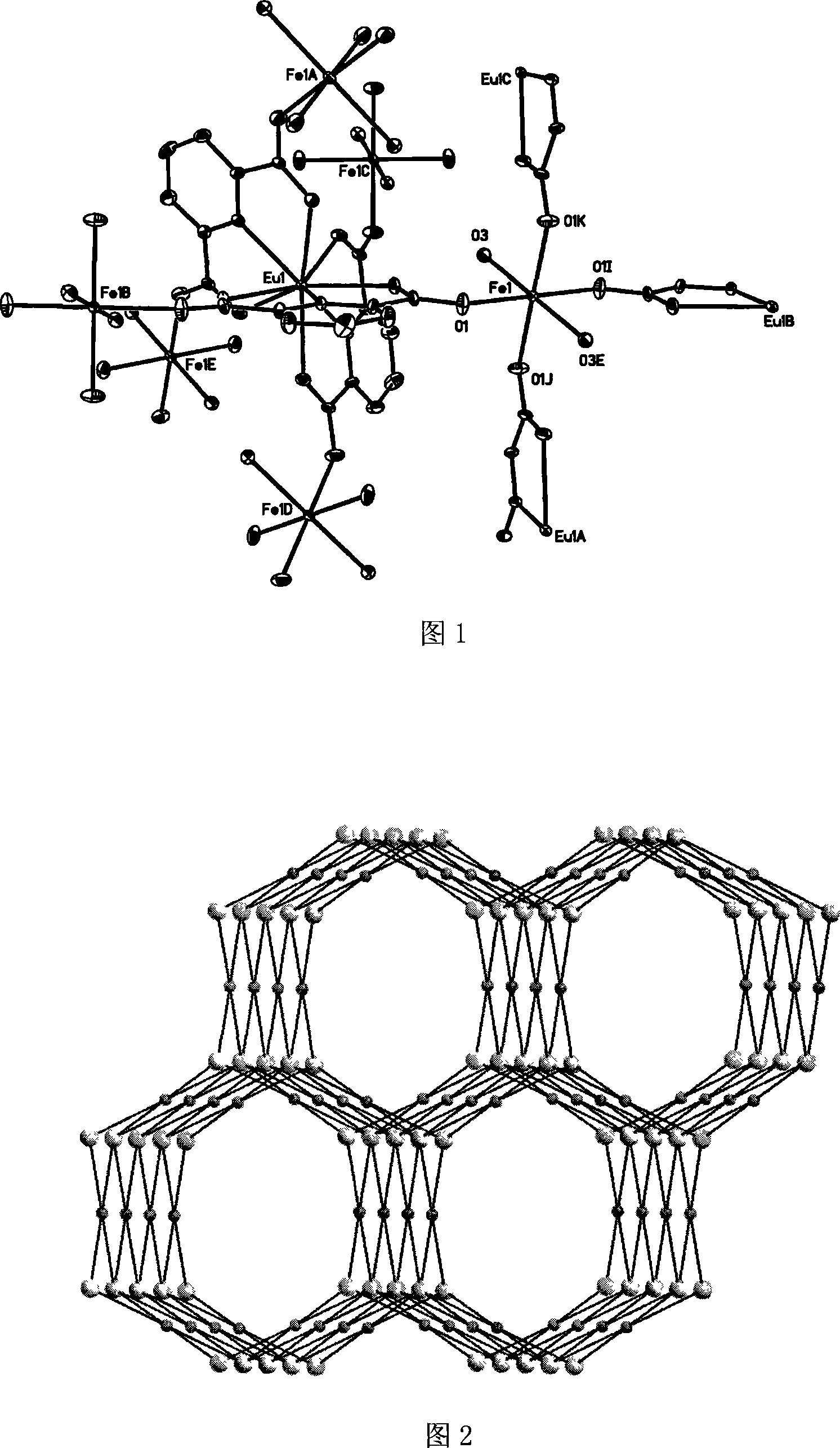
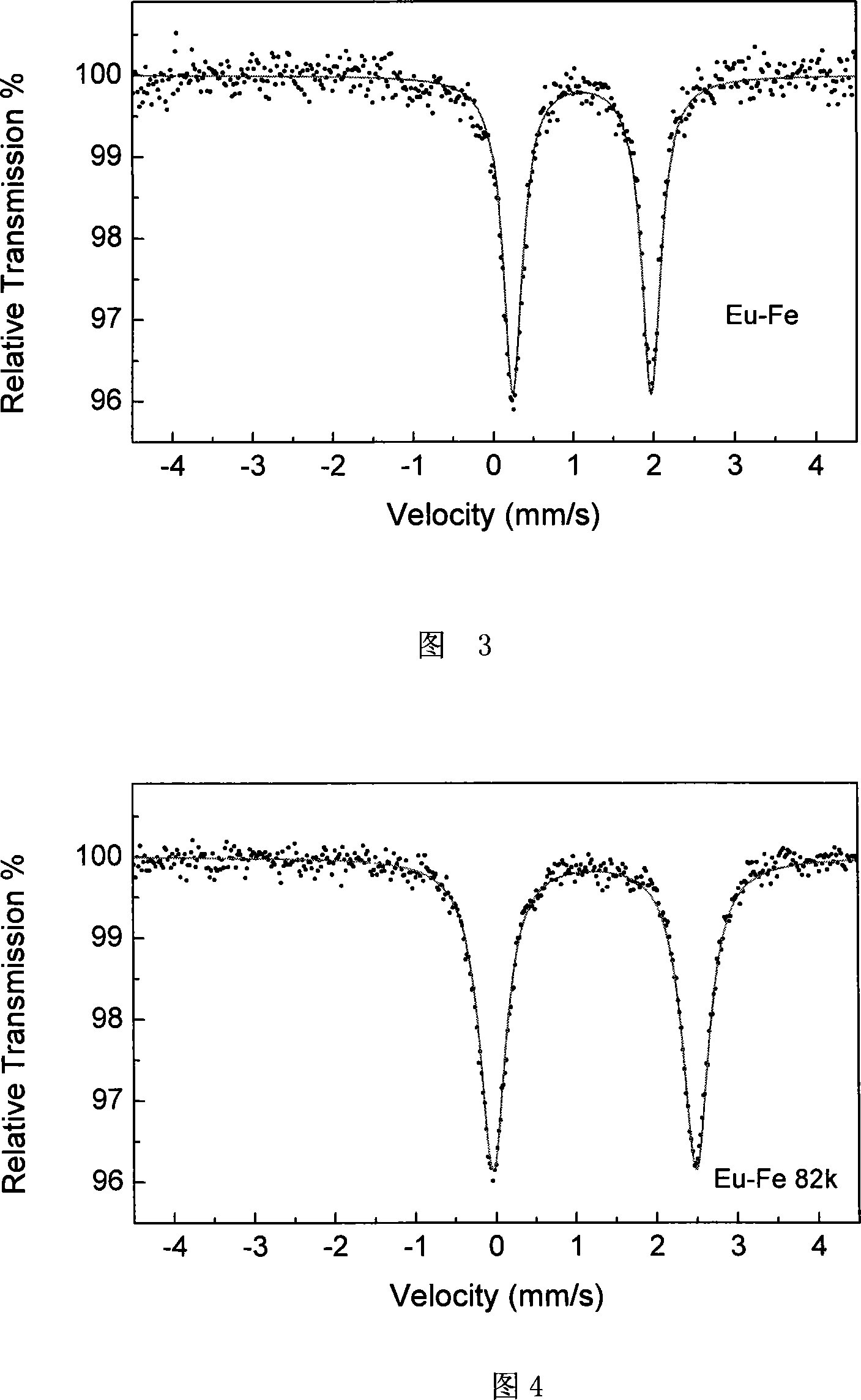
[0032] The crystal structure was determined using BRUKER SMART 1000 X-ray diffractometer, using graphite monochromatized Mokα rays (λ=0.71073 Ȧ) as the incident radiation, collecting diffraction points by ω-φ scanning, and correcting the unit cell by least square method Parameters, the crystal structure was obtained from the difference Fourier electron density map using the SHELXL-97 direct method, and was corrected by Lorentz and polarization effects. All H atoms were synthesized by difference Fourier and determined by ideal position calculation. The detailed crystal determination data are shown in Table 1. The structure is shown in Figure 1 and Figure 2.
[0033] Table 1 [Eu(PDA) 3 Fe 1.5 (H 2 O) 3 ]·1.5H 2 Crystallographic data for O
[0034] Empirical formula
Identification code
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com