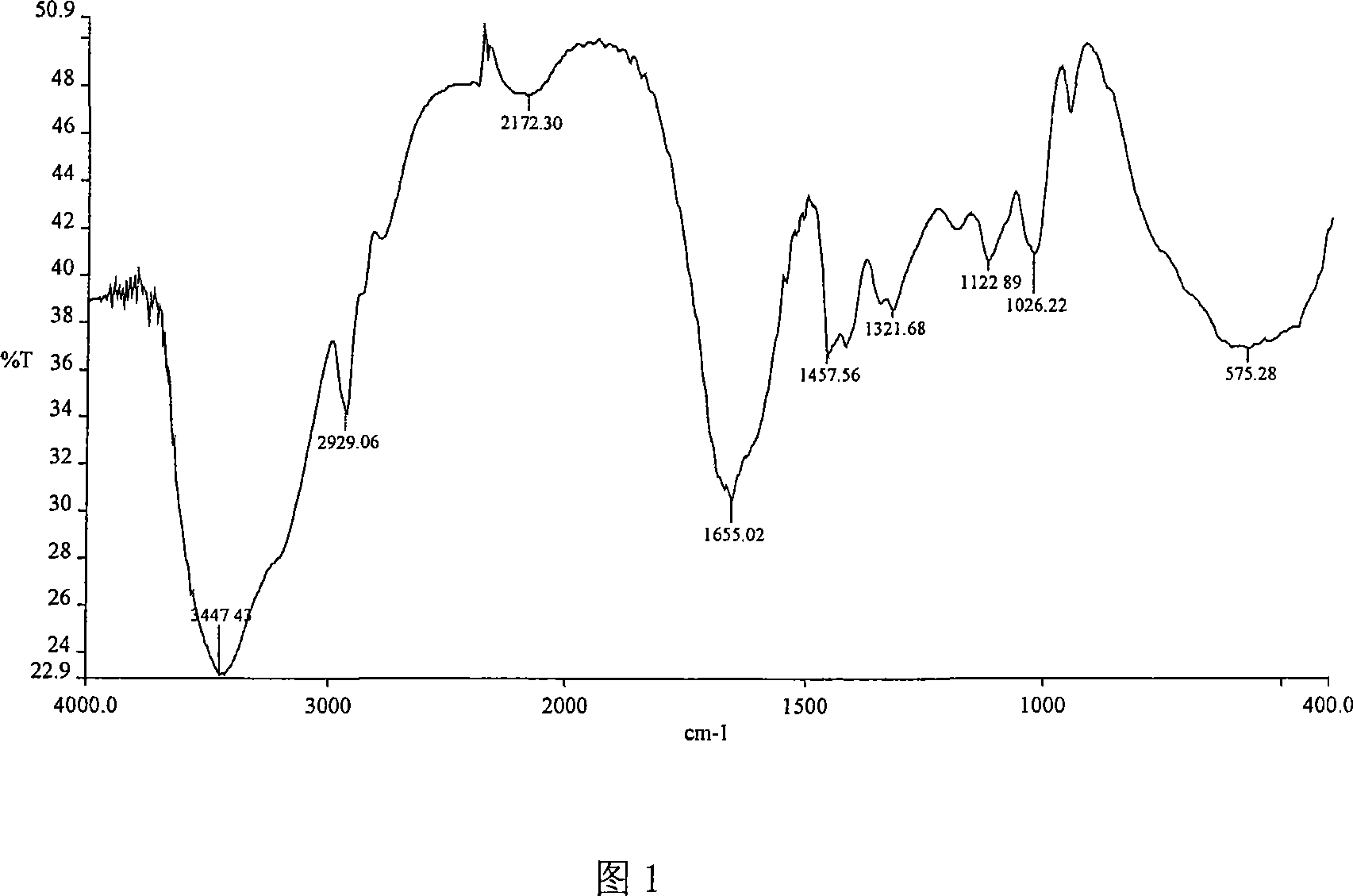
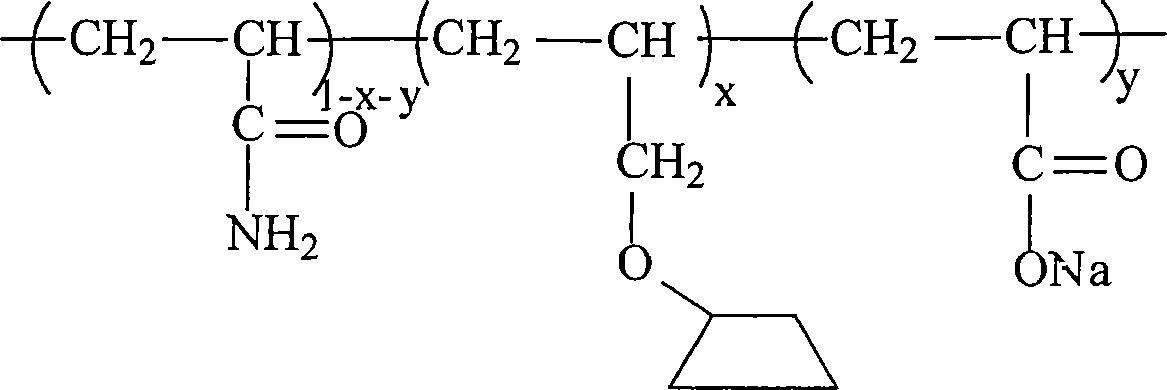
AM/NaAA/allylcyclodextrin polymer with inclusion function and synthetic method thereof
A technology of allyl cyclodextrin and synthesis method, which is applied in drilling compositions, chemical instruments and methods, water/sludge/sewage treatment, etc. Limitations and other problems, to achieve the effect of easy purification and separation, simple and feasible preparation method, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Synthesis of Example 1 Allyl Cyclodextrin
[0019] Put 5.7g (5mmol) β-CD in a beaker, add 30mL DMSO and 50mL DMF to dissolve it, then add 2.0g NaOH, place it on a magnetic stirrer and stir it well for about 1h. The color of the solution gradually deepened and turned yellow, and the pH value was 9 at this time. At 0°C to 10°C, 8.0 mL of propene bromide was slowly added dropwise twice, and stirred with a magnetic stirrer, the pH value dropped to 7, the color of the solution became light yellow, and precipitation began to appear after 24 hours of reaction. After 48 hours the reaction was stopped and a white precipitate was obtained. DMSO was removed by vacuum filtration, and the precipitate was washed with acetone to obtain 6.10 g (yield: 72.6%) of white powder, which was sealed and stored in cold storage.
example 2
[0020] Example 2 has the binary polymer composition of inclusion function
[0021] Weigh the allyl cyclodextrin monomer and distilled water according to the ratio in Table 2, put them in a 100mL Erlenmeyer flask, put them in a constant temperature water bath at 40°C to dissolve them completely, then weigh acrylamide and add them into the solution, and wait for it to dissolve completely , through N 2 After about 20 minutes, add the pre-prepared ammonium persulfate solution (8 mg / mL) and continue to pass nitrogen gas for 10 minutes, then seal and react at a constant temperature of 45°C for 8 hours, precipitate with absolute ethanol, and dry under vacuum at 40°C to obtain the polymer.
[0022] Table 2 binary polymer preparation
[0023] medicine
example 3 2
[0024] Example 3 binary copolymers are hydrolyzed into ternary polymer synthesis
[0025] In a 250mL Erlenmeyer flask, add 0.5g of the binary copolymer synthesized in the above example and 100mL of distilled water. After the polymer is completely dissolved, add the hydrolysis agent NaOH according to Table 3, react at 50°C for 4h, and precipitate the polymer with acetone. , and then vacuum-dried at 40°C to obtain terpolymers with different degrees of hydrolysis, see Table 3 for details.
[0026] Table 3 Terpolymer hydrolysis degree
[0027] NaOH(g)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com