Refractory metal prolease gene engineering bacterium and acquiring method therefor
A technology of metalloprotease and high temperature resistance, applied in genetic engineering, microorganism-based methods, plant gene improvement, etc., can solve the problems of low enzyme activity, unstable expression of high temperature resistance, unstable enzyme production, etc., and achieve easy purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Amplification and validation of Bacillus Licheniformis CGMCC NO0800 high-temperature metalloprotease.
[0071] By designing a pair of primer sequences: EMP1 ATA CGG GTG GTT GAC ACT
[0072] EMP2ACA TGA ATA TGG CTA GTT TGC
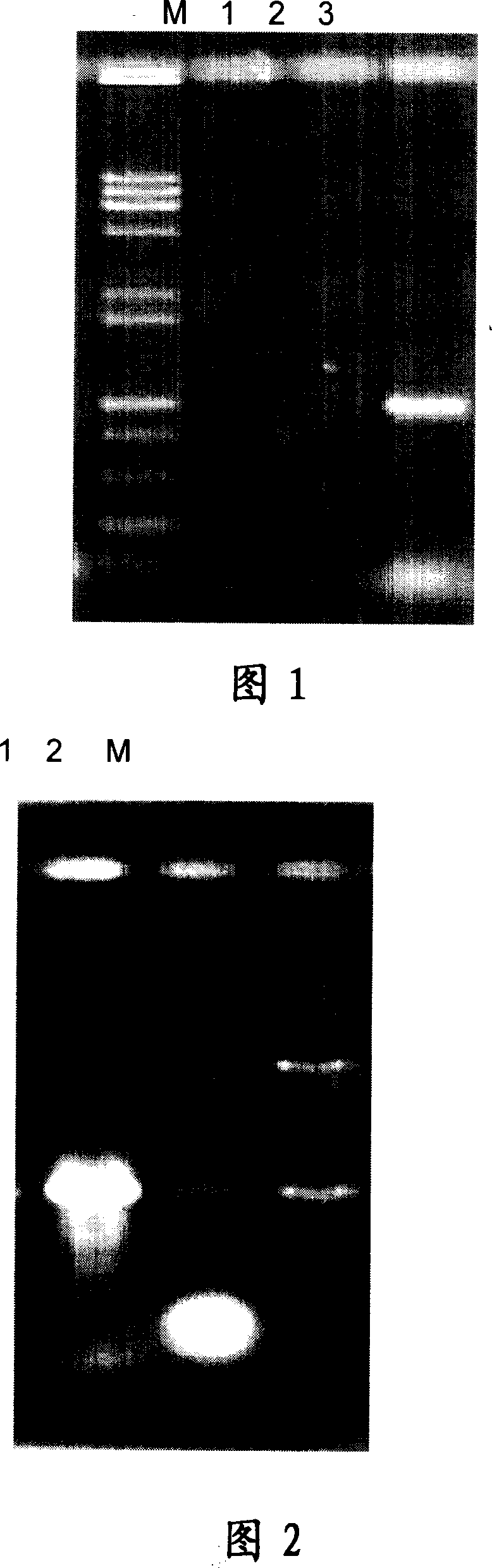
[0073] Using the chromosome of XJT9503 bacteria as a template, a 1005bp fragment EMP was amplified by PCR, and the PCR reaction was carried out in a 50μl system. Take 1 μl of DNA extracted by XJT9503 as a template, add 20 μl of sterilized deionized water, 25 μl of Taq enzyme mixed solution, EMP12 μl (25 pmol), EMP22 μl (25 pmol), and amplify the high-temperature neutral protease gene in a PCR automatic cycler, cycle parameters Denaturation at 94°C for 5 min, 94°C for 1 min, 55°C for 1 min, 72°C for 2 min, 30 cycles, and finally 72°C for 10 min. After the cycle, take 5 μl of the PCR product for 1% agarose gel electrophoresis, ethyl bromide After ingot staining, observe under ultraviolet light and analyze PCR products, see Figure 1.
[00...
Embodiment 2
[0085] Embodiment 2: Transformation of high temperature resistant metalloprotease gene
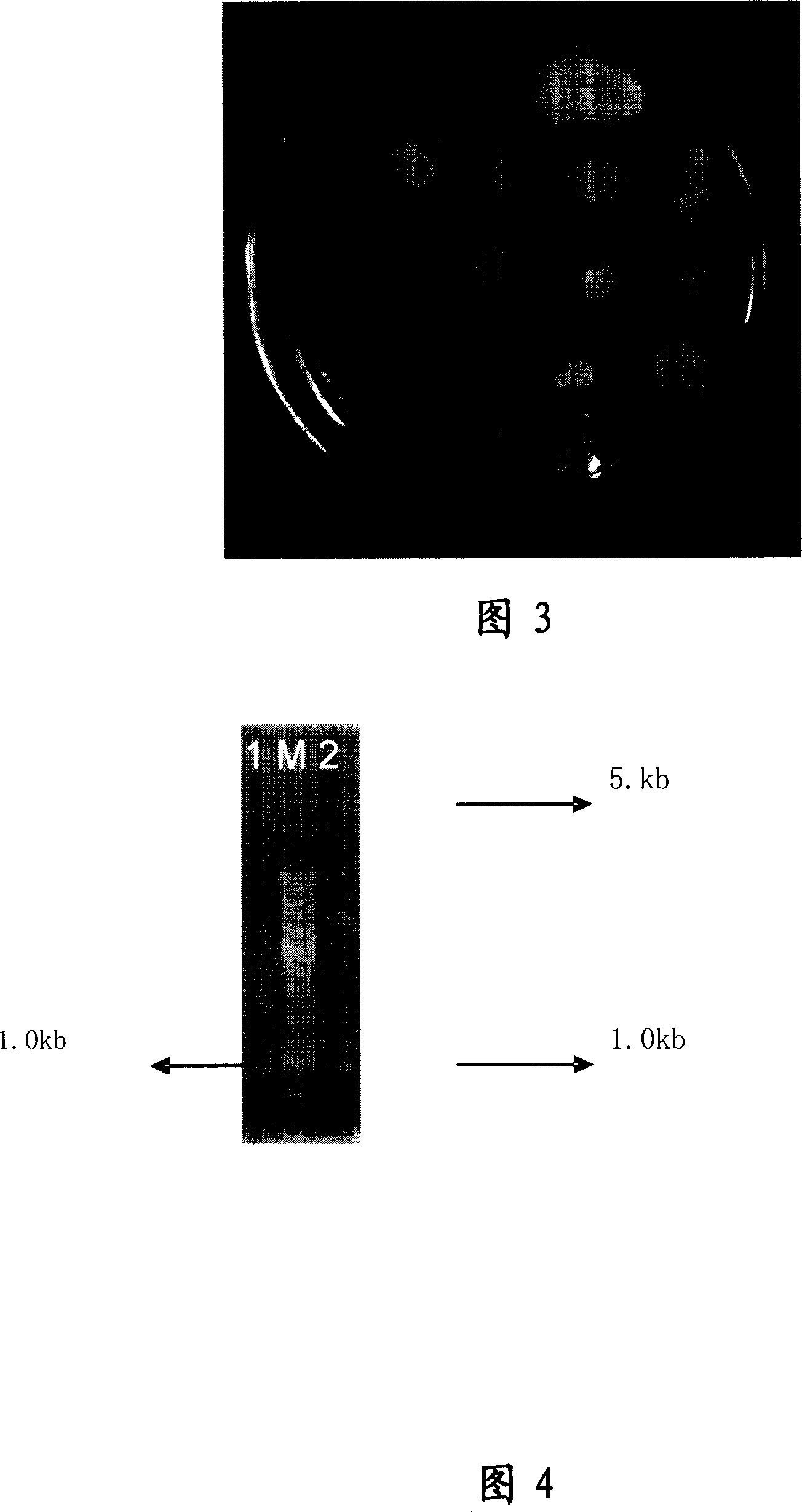
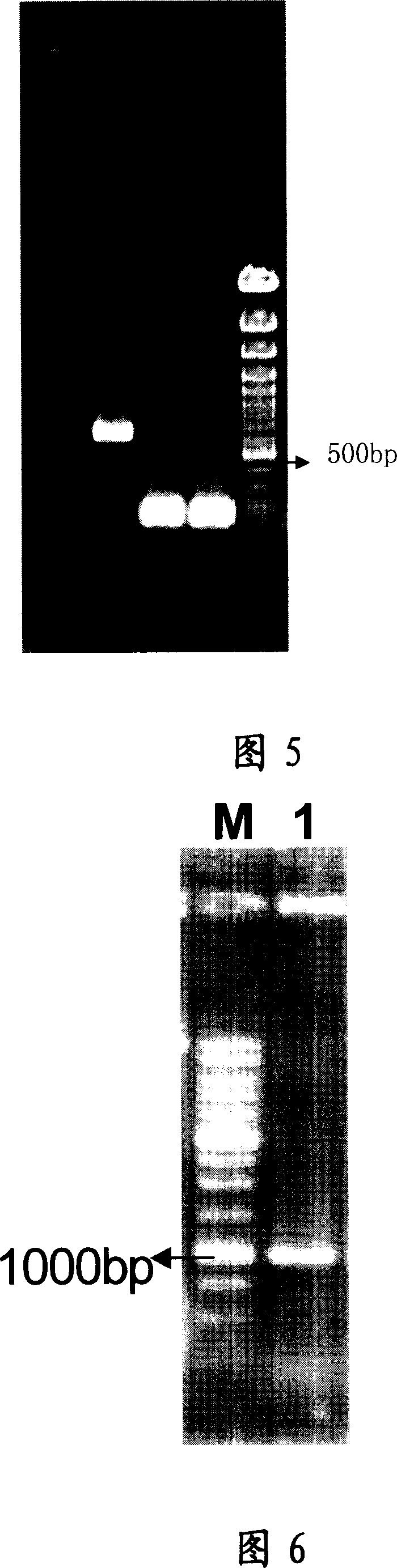
[0086] According to the codon preference of yeast, the obtained genes were analyzed and the codons encoding some amino acids were found to have low expression or even zero expression. Three pairs of primers were designed to perform site-directed mutagenesis on these codons to transform the gene, see Figure 5, Figure 6; Refer to Figure 7 for the transformation plan.
[0087] P1: GCACGAATTCATACGGGTGGTTGACACT
[0088] P2: TGTAGTACGGTAGCCGTACCAG
[0089] P3: GGCTACCGTACTACAAACAGCAGC
[0090] P4: GTTACGATAAACAGGCGAGCCGCTTTG
[0091] P5: CCTGTTTATCGTAACTACAGTGATACAGG
[0092] P6: GTACGCGGCCGCACATGAATATGGCATGTTTGC
[0093] Use primers P1, P3 paired, P4, P5 paired, P6, P2 paired with plasmid pMD18-TE as a template for PCR, denaturation at 94°C for 5min, 94°C for 1min, 55°C for 1min, 72°C for 2min, and the number of cycles is 30 , recover the above-mentioned three-stage PCR product, and then ...
Embodiment 3
[0094] Example 3: Construction of Yeast Expression Vector and Acquisition of Genetically Engineered Bacteria Containing Thermostable Metalloprotease Gene
[0095] The modified gene was digested with EcorI and NotI and connected with the yeast expression vector pPIC9K vector with EcorI and NotI to construct vector 9TE. After identification, the sequencing results proved that the gene transformation was successful and the reading frame was correct, see Figure 8 and Figure 9.
[0096] The constructed yeast expression vector, SacI linearized CIAP dephosphorylated, electrotransformed protease-deficient yeast strain SMD1168, the transformation method is as follows:
[0097] 1. Take a single colony of SMD1168 in 5ml YPD culture medium, shake overnight at 30°C, take 20ul of the cultured bacteria into 100ml YPD culture medium, culture at 30°C until the cell density reaches 1-2A OD 600 . According to the formula 12A OD600=5×10 7 cell / ml Count cells.
[0098] 2. Collect the cells in 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com