Rubrene derivatives and preparation method thereof
A technology of derivatives and rubrene, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve the problems of concentration quenching effect and low fluorescence quantum yield, and achieve reduced concentration quenching effect and high fluorescence Quantum efficiency and the effect of improving luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
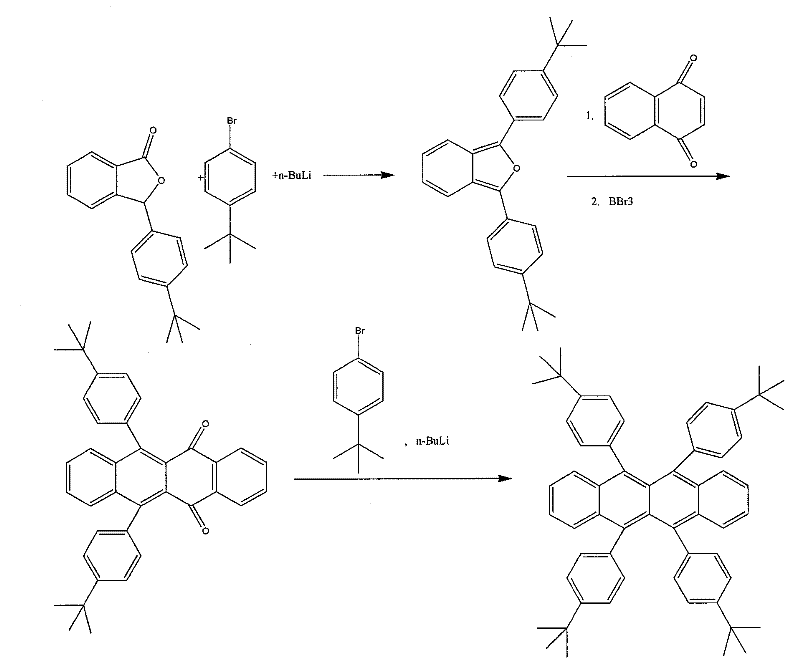
[0037] Embodiment 1: 5,6,11,12-tetra-tert-butylbenzene-naphthonaphthalene synthesis (such as figure 1 ):
[0038] (1) Synthesis of 1,3-di-p-tert-butylbenzofuran
[0039] In a 150ml four-neck flask, stir and dissolve p-tert-butylbromobenzene (4.24g, 20mmol) in 50ml THF or DMF, cool to -78°C, and add 1.6M n-butyllithium n-hexane solution through a constant pressure dropping funnel (12.5ml20mmol) was added dropwise, keeping the temperature of the mixture in the flask lower than -60°C, at -78°C, stirred for 1h, then continued to drop p-3-tert-butylphenyl-phenylpeptide (5.32 g, 20mmol) in THF or 25ml of DMF solution, the dark red mixed solution was stirred at -78°C for 15min, then added acetic anhydride (2.0ml, 20mmol) and slowly raised to room temperature, then heated to reflux for 10min, and 50ml of water was added to end the reaction. The organic layer was separated and washed with anhydrous MgSO 4 After being dried and spin-dried in vacuo, recrystallized with a mixed solvent...
Embodiment 2
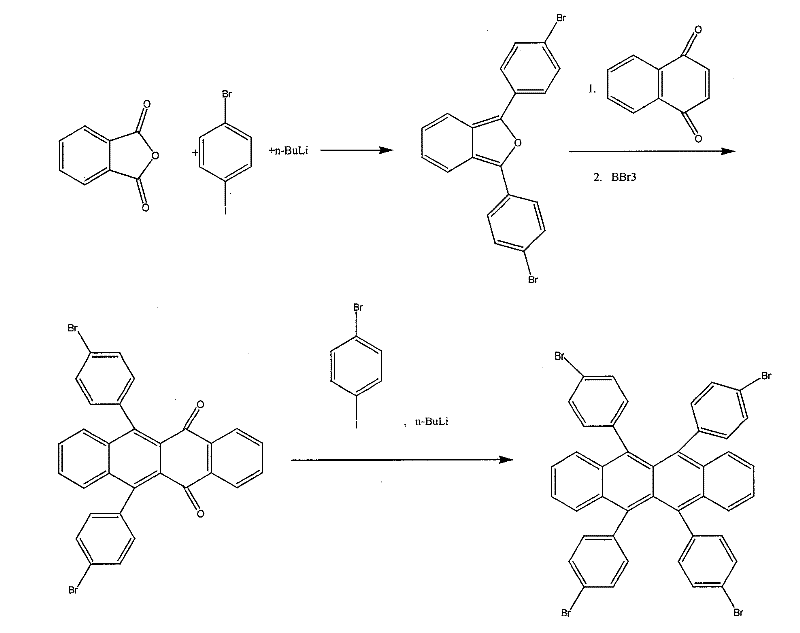
[0055] Embodiment 2: 5,6,11, the synthesis of 12-tetrabromobenzene-naphthacene (as figure 2 ):
[0056] (1). Synthesis of 1,3-di-p-bromophenylbenzofuran
[0057] In a 150ml four-neck flask, dissolve p-bromoiodobenzene (5.64g, 20mmol) in 50mlTHF, cool to -78°C, and add 1.6M n-butyllithium-n-hexane solution (12.5ml 20mmol) gradually through a constant pressure dropping funnel Drop in, keep the temperature of the mixture in the flask lower than -60°C, stir for 1h at -78°C, then continue to drop 25ml of THF solution of terephthalic anhydride (5.76g, 10mmol) in 45 minutes, deep The red mixed solution was stirred at -78°C for 15min, then added acetic anhydride (1.0ml, 10mmol) and slowly raised to room temperature, then heated to reflux for 10min, and 50ml of water was added to complete the reaction, the organic layer was separated and washed with anhydrous MgSO 4 After drying and spin-drying in vacuo, recrystallization was performed with a mixed solvent of ethanol and benzene to ...
Embodiment 3
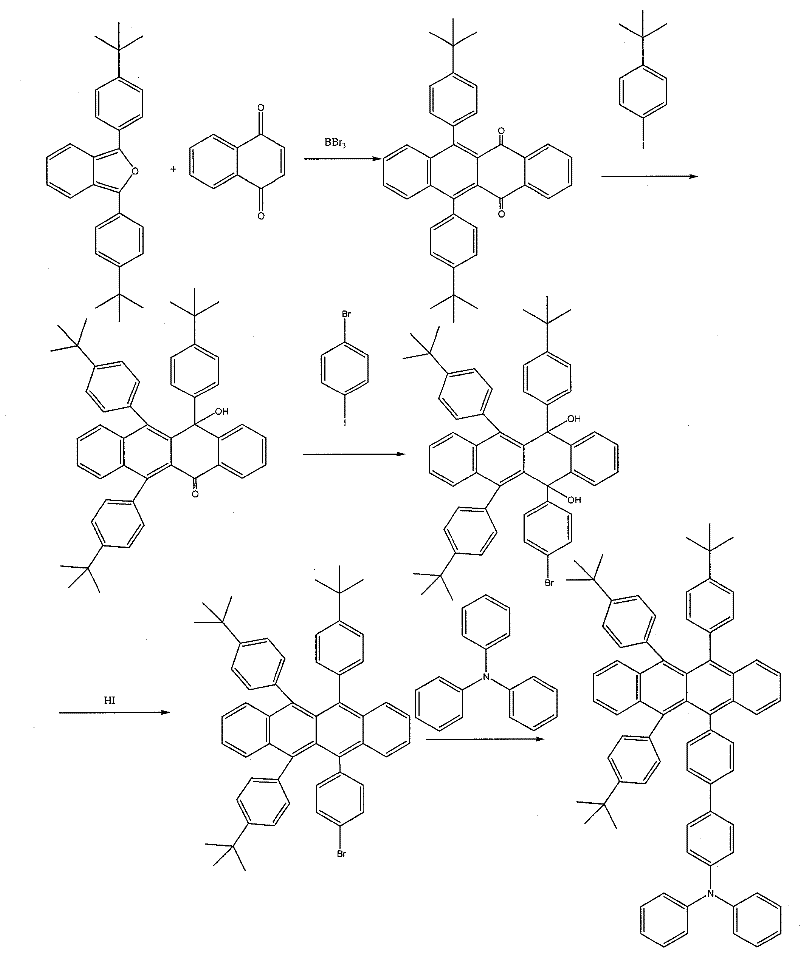
[0073] Embodiment 3: 5,11-di-tert-butylbenzene-6, the synthesis of 12-triphenylamine-naphthacene (as image 3 ):
[0074] (1) Synthesis of 5,11-p-tert-butylphenyl-naphthoquinone
[0075] In a 150ml four-necked flask, 1.3-p-tert-butylbenzofuran (0.2g, 0.5mmol) was slowly added to 5ml CH of 0.08g (0.5mmol) 1,4-naphthoquinone 2 Cl 2 solution, stirred at room temperature for 12h, continued to add 10ml CH 2 Cl 2 , cooled to -78°C, 0.58ml 1M BBr 3 CH 2 Cl 2 The solution was added dropwise and reacted at -78°C for 0.5h. The dark black reaction system was then raised to room temperature, heated to reflux for 4h, and then cooled to room temperature. The reaction solution was poured into water, and the aqueous phase was separated with CH 2 Cl 2 Extraction, combined organic phases, washed with brine, anhydrous MgSO 4 Dry and concentrate in vacuo to give a yellow oil, CHCl 3 -MeOH was recrystallized to obtain a yellow solid with a yield of 55.5%.
[0076] (2) Synthesis of 6-hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com