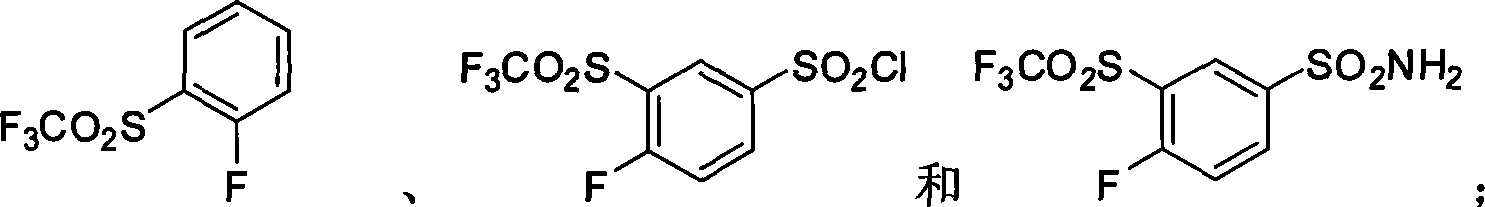
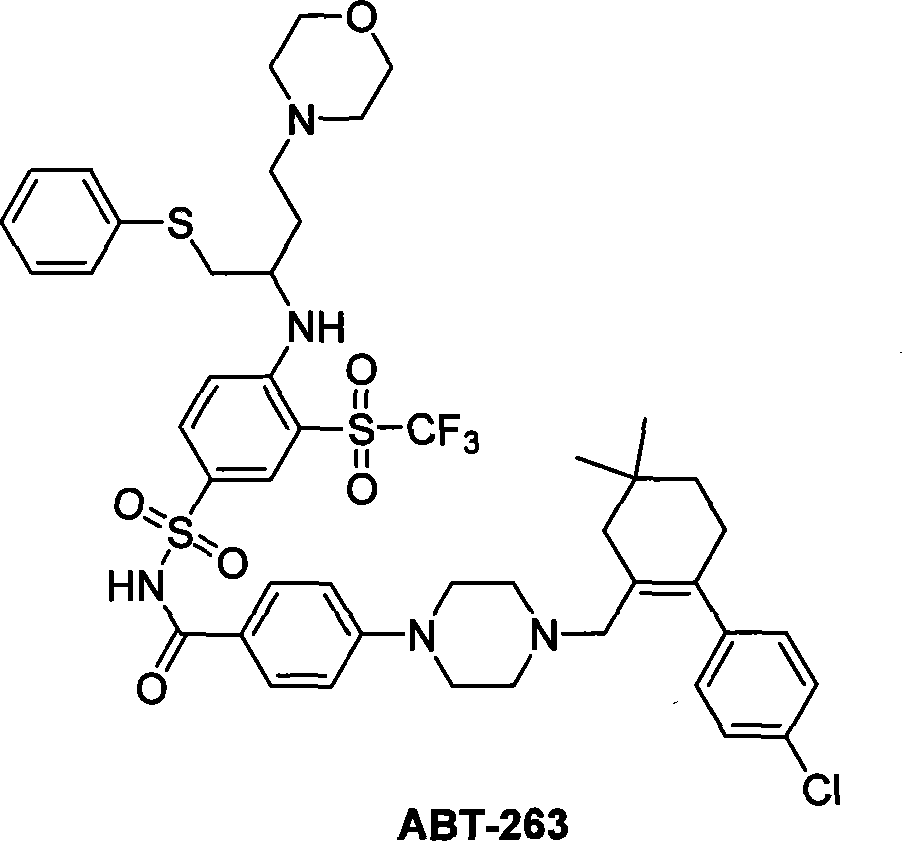
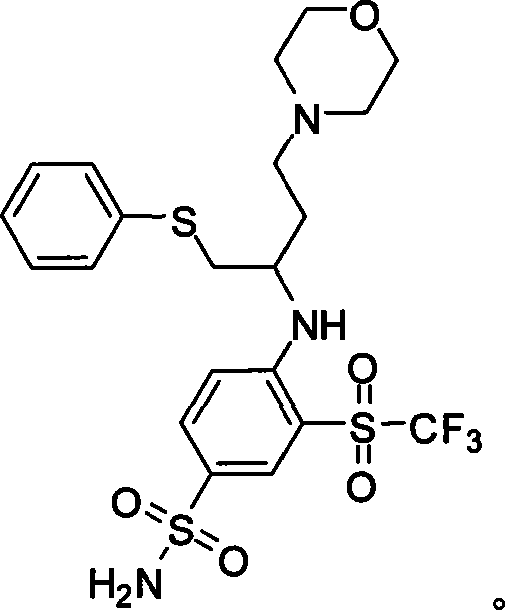
Synthesis of compound ABT-263
A technology of ABT-263 and synthetic method, which is applied in the field of synthesis of Bcl-2 and Bcl-xl inhibitor compound ABT-263, can solve problems such as difficult to prepare large quantities of compounds, shorten reaction steps, and environmental pollution, and achieve synthetic method The effect of simplicity, shortening the route, and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of compound 1
[0043]In a 500mL three-neck flask, add 200mL tetrahydrofuran, add 20mL N-methylmorphine and (R)-N-tert-butoxycarbonylaspartic acid-4-benzyl ester (32.3g, 0.1mol) at -10°C, Add tert-butyl chloroformate (16.2g, 0.12mol) dropwise under stirring, react for 30 minutes, add sodium borohydride (7.6g, 0.2mol), add methanol 150ml dropwise, stir for 2 hours; spin the organic solvent to dry, Add 150ml of water, extract with ethyl acetate (3×100mL), combine, dry, concentrate, and pass through the column to obtain compound 119.7g with a yield of 64%.
[0044] Synthesis of Compound 2
[0045] Diethyl azodicarboxylate (13g, 75mmol) and triphenylphosphine (19.6g, 75mmol) were dissolved in 150mL of dichloromethane, compound 1 (15.5g, 50mmol) was added dropwise, followed by thiophenol (8.25 g, 75 mmol), stirred at room temperature for 24 hours, spin-dried the organic solvent, and separated by column chromatography to obtain compound 2 (11.2 g, yield 56%).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com