Alpha-biphenyl-N-alkyl nitrone compound and synthetic method thereof
A technology of alkyl nitrones and compounds, which is applied in the field of α-biphenyl-N-alkyl nitrones and their synthesis, can solve the difficulties of free radical detection, short half-life of spin adducts, free radical adducts Slow formation speed and other problems, to achieve the effect of increasing steric hindrance, increasing lipophilicity, and weakening enzyme catalytic quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
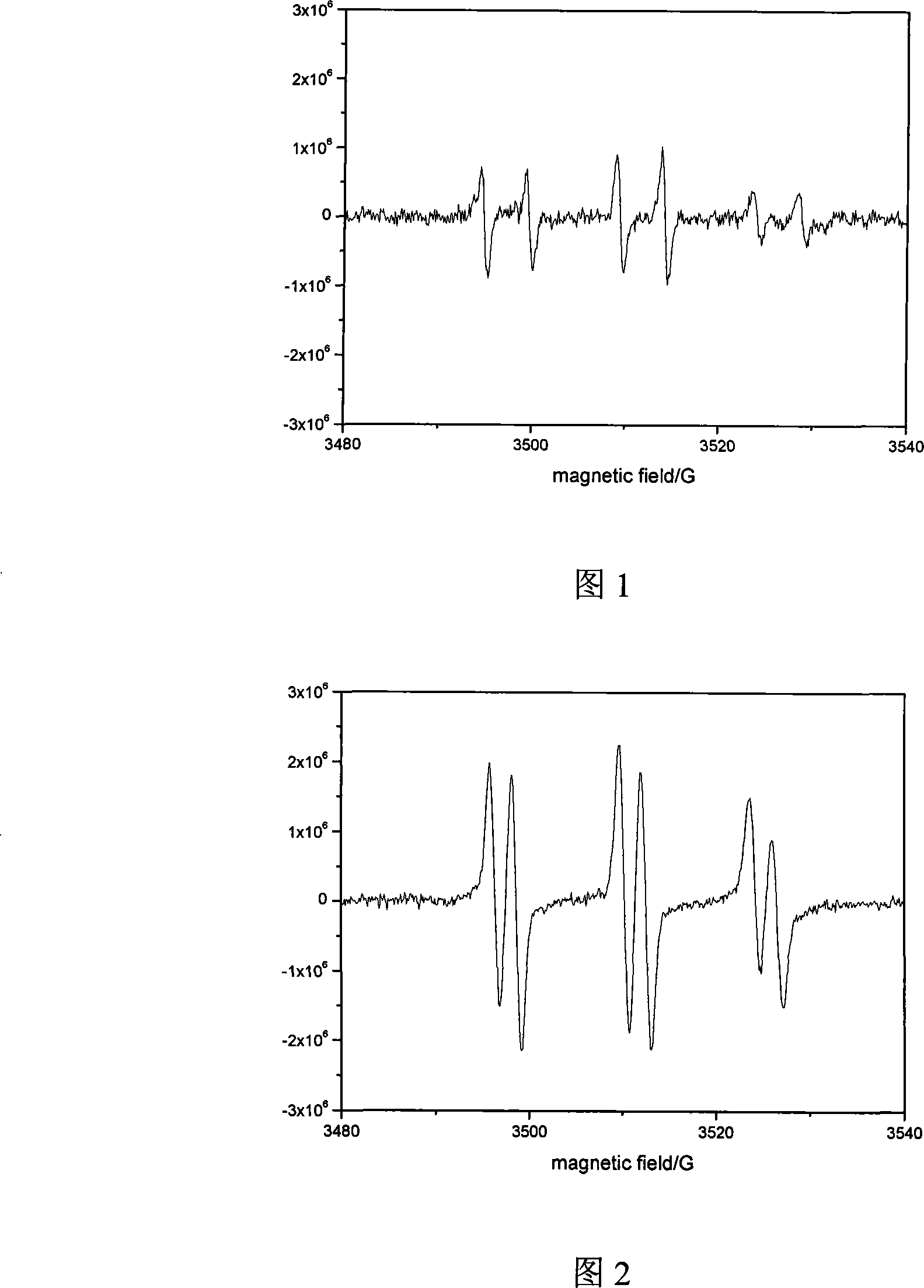
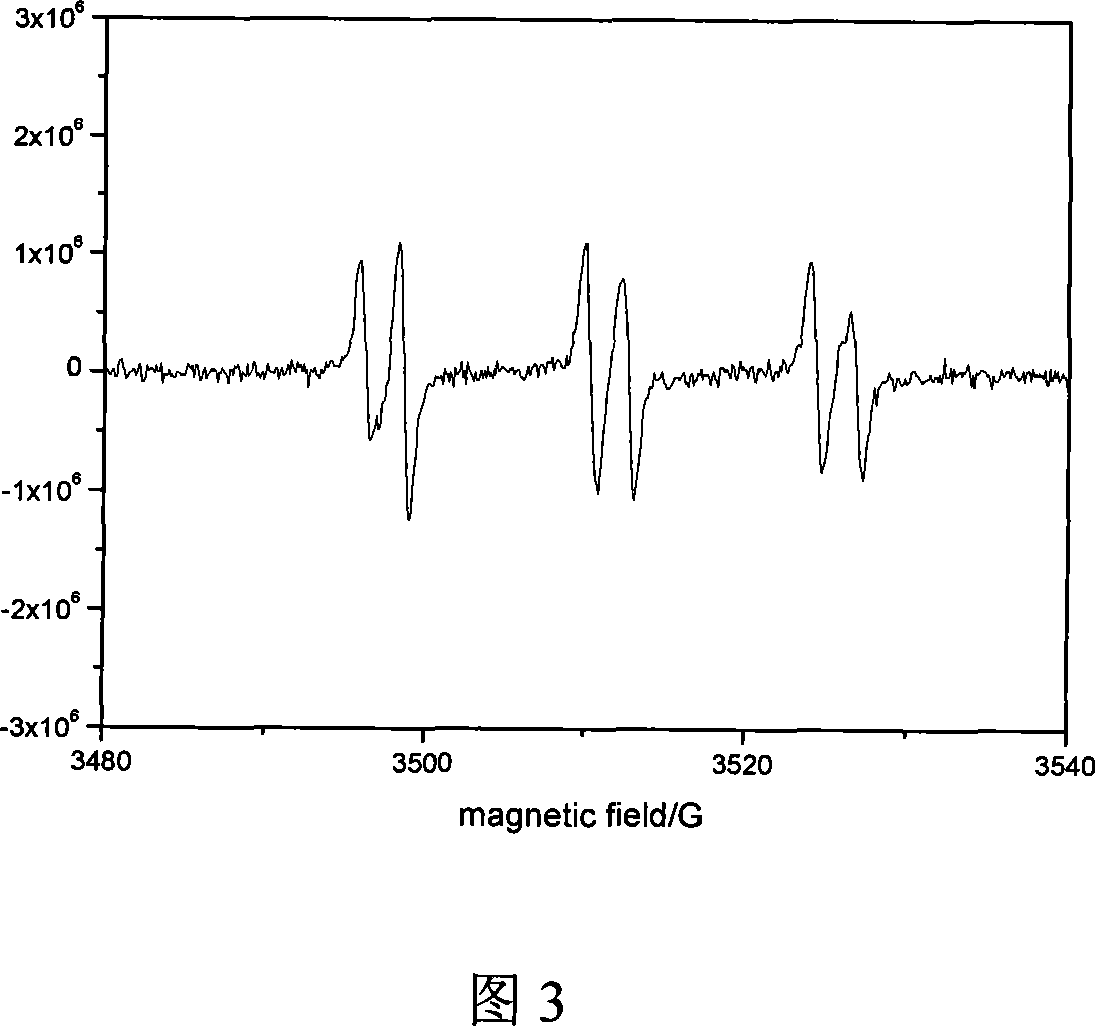
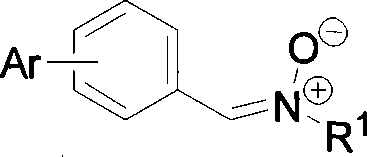
Image
Examples
Embodiment 1
[0056] The synthesis of embodiment 1α-(2-phenyl) phenyl-N-tert-butylnitrone
[0057] 1) Synthesis of 2-phenylbenzaldehyde: add 1.85 grams (10mmol, 1.0eq) of 2-bromobenzaldehyde, 1.83 grams (15mmol, 1.5eq) of phenylboronic acid, 6.9 grams (30mmol, 3.0 eq) of potassium phosphate hydrate, 0.58 g (5mol%) of Pd(Ph 3 P) 4 and 50 mL of toluene, heated to 80° C. for 2.5 hours under nitrogen protection. After the reaction, lower the temperature to room temperature, add 50mL of water and stir for 5 minutes, extract with ethyl acetate (50mL×3), combine the organic phases, and successively use 10mL of 1N sodium hydroxide aqueous solution, 100mL of water and 100mL of saturated saline Wash each time. The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography (ethyl acetate / petroleum ether=1 / 5) to obtain 1.78 g of 2-phenylbenzaldehy...
Embodiment 2
[0059] Example 2 Synthesis of α-[2-(2-methylphenyl)]phenyl-N-tert-butylnitrone
[0060] 1) Synthesis of 2-(2-methylphenyl)benzaldehyde: replace the phenylboronic acid in Example 1 with 2.04 grams (15mmol, 1.5eq) of 2-methylphenylboronic acid, with 1.9 milligrams (0.02mol%) Pd 2 (dba) 3 +0.8 milligrams of N,N-diisopropyl-2-dicyclohexylphosphine benzamide instead of Pd(Ph 3 P) 4 , the reaction temperature is 60 DEG C, reacted for 20 hours and separated and purified according to the method of Example 1 to obtain 0.78 g of 2-(2-methylphenyl)benzaldehyde, with a yield of 40%. 1 HNMR (400MHz, CDCl 3 ): δ9.78(s, 1H), 8.05(d, J=8.0Hz, 1H), 7.64(t, J=7.6Hz, 1H), 7.51(t, J=7.2Hz, 1H), 7.36-7.27 (m, 4H), 7.20 (d, J=7.2Hz, 1H), 2.12 (s, 3H).
[0061] 2) Synthesis of α-[2-(2-methylphenyl)]phenyl-N-tert-butylnitrone: with 0.392 grams (2mmol, 1.0eq) of 2-(2-methylphenyl)benzene Formaldehyde replaces the 2-phenylbenzaldehyde in Example 2, and reacts and separates and purifies according...
Embodiment 3
[0062] Example 3 Synthesis of α-[2-(4-methylphenyl)]phenyl-N-tert-butylnitrone
[0063] 1) Synthesis of 2-(4-methylphenyl)benzaldehyde: replace phenylboronic acid in Example 1 with 2.04 grams (15mmol, 1.5eq) of 4-methylphenylboronic acid, and react for 12 hours according to Example 1 method And separation and purification treatment, 1.52 grams of 2-(4-methylphenyl)benzaldehyde was obtained, with a yield of 78%. 1 H NMR (400MHz, CDCl 3 ): δ9.96(s, 1H), 8.02(d, J=8.8Hz, 1H), 7.62(t, J=7.6Hz, 1H), 7.49-7.43(m, 2H), 7.28(s, 4H) , 2.43(s, 3H).
[0064] 2) Synthesis of α-[2-(4-methylphenyl)]phenyl-N-tert-butylnitrone: 0.392 grams (2mmol, 1.0eq) of 2-(4-methylphenyl)benzene Formaldehyde replaces the 2-phenylbenzaldehyde in Example 2, and reacts and separates and purifies according to the method of Example 2 to obtain α-[2-(4-methylphenyl)]phenyl-N-tert-butylnitrone 0.16 g, 30% yield. MS(ESI): m / z 268.1(M+H); 1 H NMR (400MHz, CDCl 3 ): δ9.31(d, J=6.8Hz, 1H), 7.48(s, 1H), 7.40-7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com