Fusion protein for serum albumin and interleukin 1 receptor antagonist and uses thereof
A receptor antagonist, serum albumin technology, applied in the fusion protein field of serum albumin and interleukin 1 receptor antagonist, can solve the problems of toxic and side effects, increased patient pain and treatment costs, short plasma half-life, etc., to achieve The effect of prolonging the half-life, benefiting the development of industrialization, and reducing the frequency of injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Cloning of HSA cDNA
[0038] HSAcDNA without signal peptide coding sequence was obtained from human liver fetal cDNA library by PCR method, and the primers HSA up (SEQ ID NO: 1) and HSA dn (SEQ ID NO: 2) were synthesized with oligonucleotides The upstream and downstream primers were respectively introduced into EcoRI and BamHI sites and protective bases, and the underlined part was the endonuclease recognition sequence.
[0039] HSA-up: 5'ATGC GAATTC GATGCACACAAGAGTGAGGTT 3'
[0040] HSA-down: 5'ATGC GGATCC TAAGCCTAAGGCAGCTTGACT 3’
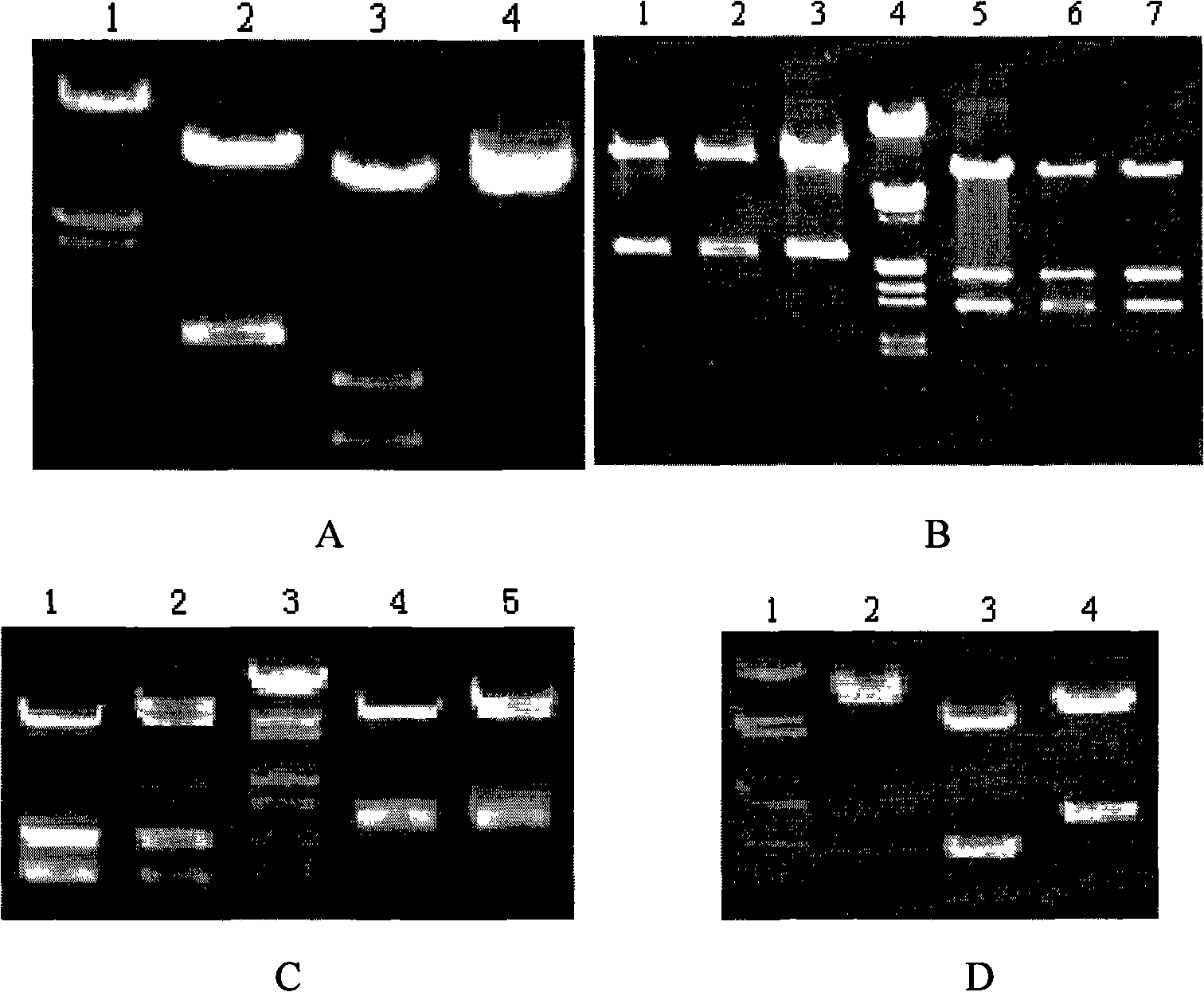
[0041] PCR reaction conditions: In 100 μL reaction system, add 1.5 μL liver tissue cDNA, 20 μmol / L upstream and downstream primers 1.5 μL each, 10 mmol / L dNTP (deoxynucleotide) 1 μL, 10× reaction buffer 10 μL, Taq DNA polymerization Enzyme 0.5 μL, the rest with ddH 2 O make up. Use EPPENDORF company (model Matstercycler Gradient) PCR instrument, PCR reaction conditions are 94°C pre-denaturation for 5 minutes; 94°C denatur...
Embodiment 2
[0043] Example 2: Cloning of IL1ra cDNA
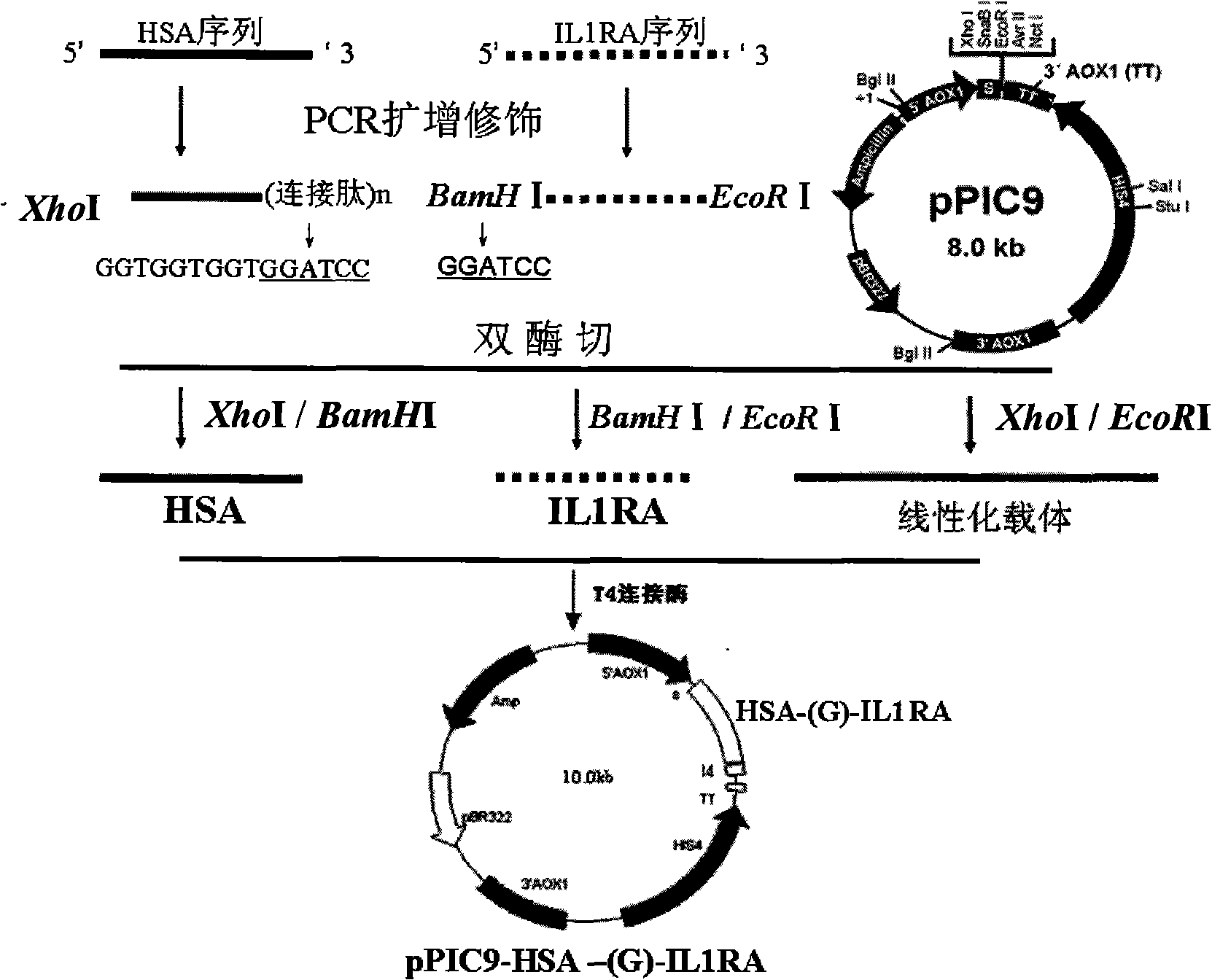
[0044] The IL1ra cDNA gene sequence was obtained from human liver by RT-PCR method, and the primers IL1raup (SEQ ID NO: 3) and IL1ra dn (SEQ ID NO: 4) used were synthesized with an oligonucleotide synthesizer, and IL1ra up 5 A BamHI restriction site was added to the 'end, so that the HSA gene and the IL1ra gene could be fused by BamHI digestion and ligation. An EcoRI restriction site was designed at the 5' end of IL1ra dn for ligation with the selected pPIC9 vector. The underline is the endonuclease recognition sequence.
[0045] IL 1ra up: 5′CC GGATCC CG AC CC TCTG GG AGAAAATC-3′
[0046] IL 1ra dn: 5′-GCA GAATTC CTACTCGTCCTCCTGGA-3′
[0047] PCR reaction conditions: In 100 μL reaction system, add 1.5 μL liver tissue cDNA, 20 μmol / L upstream and downstream primers 1.5 μL each, 10 mmol / L dNTP (deoxynucleotide) 1 μL, 10× reaction buffer 10 μL, Taq DNA polymerization Enzyme 0.5 μL, the rest with ddH 2 O make up. Use EPPENDORF...
Embodiment 3
[0049] Example 3: Expression of fusion proteins without linker peptides
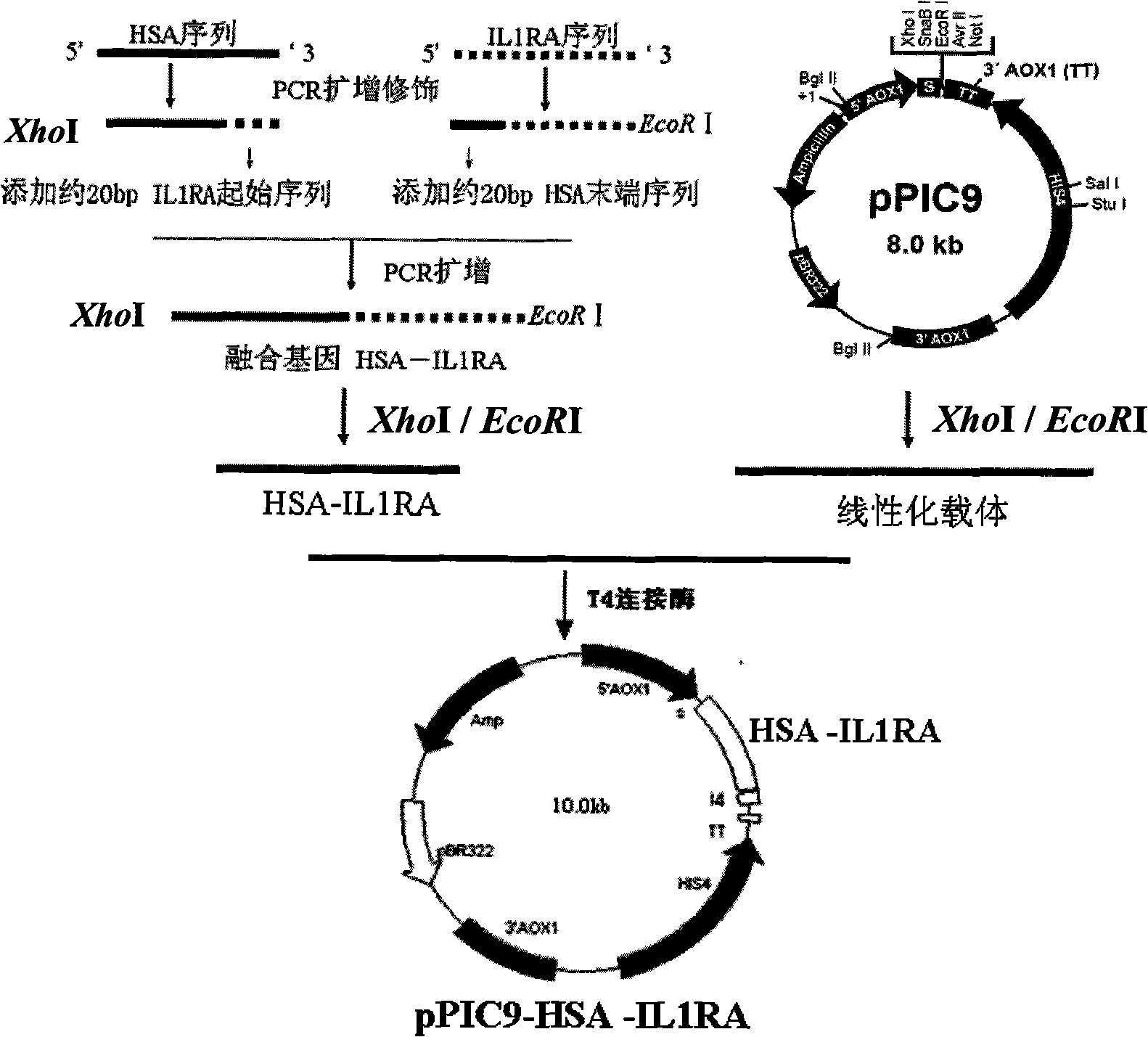
[0050] For the secreted expression of HSA and IL1ra as a directly linked fusion protein from Pichia pastoris, see figure 1 , select the pPIC9 plasmid as the vector (Invitrogen Corp. USA), and insert the fusion protein gene between the XhoI and EcoRI sites downstream of the AoX promoter of the vector.
[0051] Obtain the modified HSA, the cDNA of IL1ra from the PGEM-T-HSA, PGEM-T-IL1ra that embodiment 1,2 constructs with the PCR method similar to embodiment 1,2, used primer is changed into HSA 1 (SEQ ID NO: 5), HSA 2 (SEQ ID NO: 6) and IL1ra 1 (SEQ ID NO: 7), IL1ra2 (SEQ ID NO: 8).
[0052] HSA 1: 5'-GC ctcgag(XhoI)AAAAGA GATGCACACAAGAGTGAGG-3'
[0053] HSA 2: 5'-GGATTTTCTCCCAGAGGGTCG (IL 1ra sequence)
[0054] TAAGCCTAAGGCAGCTTGAC(HSA sequence)-3'
[0055] IL1ra1: 5′-GTCAAGCTGCCTTAGGCTTA (HSA sequence)
[0056] CGACCCTCTGGGAGAAAATCCAGCAA (IL1ra sequence)-3'
[0057] IL1ra 2: 5'-GCA gaattc(EcoRI)CTA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com