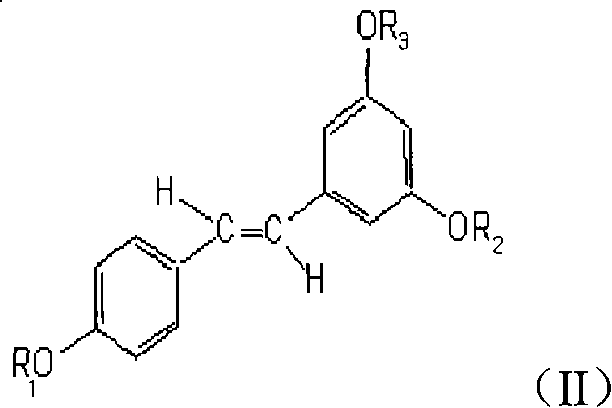
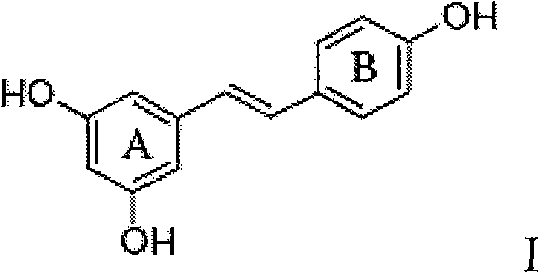
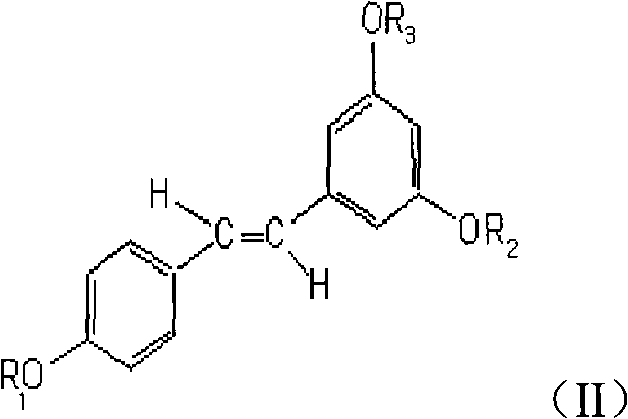
Application of resveratrol derivative in preparing medicine for treating disease relative to immune
A technology for immune-related diseases and drugs, applied in the field of treatment of immune-related arthritis, can solve the problems of slow onset and high toxicity, and achieve the effects of short half-life, low bioavailability, and strong efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Resveratrol Methylated Derivatives (BTM)
[0028] It can be prepared according to the methods disclosed in the background technology, and can also be prepared by the following methods.
[0029] Stir and dissolve 50 g of resveratrol in 350 mL of acetone, add 70 mL of dimethyl sulfate and 85 g of anhydrous potassium carbonate, let stand at 30-40°C for 24 hours, and stir intermittently. After the reaction was completed, it was poured into water and extracted three times with ethyl acetate. The ethyl acetate extracts were combined, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a crude product. 40 g of the crude product was dissolved in chloroform, stirred with silica gel and waited to dry, packed on top of a silica gel column for column chromatography, and eluted with petroleum ether-chloroform at a volume ratio of 8:2. The resveratrol methylated derivatives were collected and combined, the solvent was dist...
Embodiment 2
[0031] Preparation of acetylated derivatives of resveratrol (BTY).
[0032] It can be prepared according to the methods disclosed in the background technology, and can also be prepared by the following methods.
[0033] 60g of resveratrol was stirred and dissolved in 180mL of anhydrous pyridine, 225mL of acetic anhydride was added, and stood at 35-45°C for 24 hours with intermittent stirring. After the reaction was completed, it was poured into water and extracted with ethyl acetate. The ethyl acetate extracts were combined, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a crude product. 56 g of the crude product was dissolved in chloroform, stirred with silica gel and waited to dry, then packed on the top of a silica gel column for column chromatography, and eluted with petroleum ether-chloroform with a volume ratio of 8:3. Collect and combine the fractions of resveratrol acetylated derivatives, distill off the solvent under...
Embodiment 3
[0035] Effects of Resveratrol Acetylated Derivatives (abbreviated as BTY) on Experimental Osteoarthritis in Rabbits
[0036] Materials and Methods:
[0037] Experimental animals: Experimental animals: 36 healthy New Zealand rabbits (provided by the Experimental Animal Laboratory of the Second Xiangya Hospital of Central South University), male and female, weighing 1.5-2 kg. Certificate of Conformity: SCXK (Xiang) 2003-0003
[0038] Experimental drugs: BTY (made by Central South University, purity 98.5%), glucosamine sulfate (positive drug, Zhejiang Hisun Pharmaceutical Co., Ltd., 0504081). Reagent: According to the conversion formula of dosage for adults and dosage for experimental animals, calculate the high dose of BTY for each rabbit every day 120mg / kg, the medium dose of 60mg / kg, and the low dose of 30mg / kg. Glucose 35mg / kg was made into a solution with a concentration of 35mg / mL.
[0039] Experimental instruments: BECKMAN spectrophotometer, NiKonYS700 microscope, LEICA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com