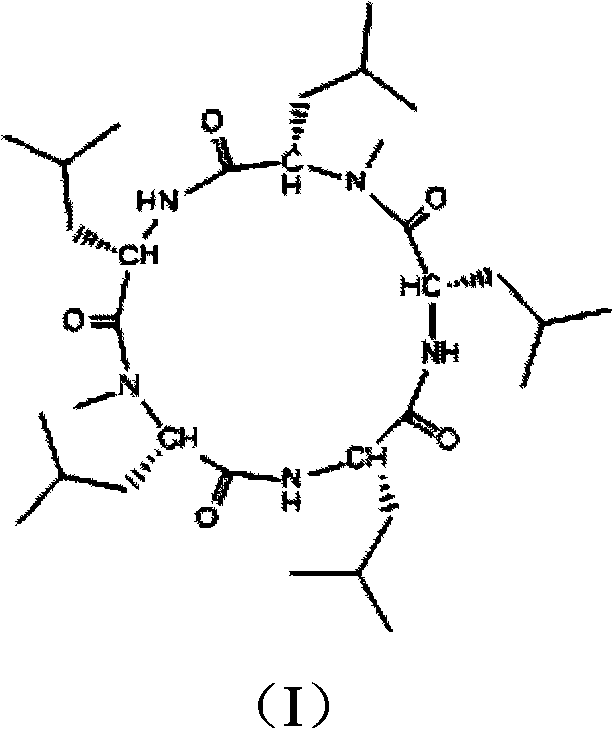
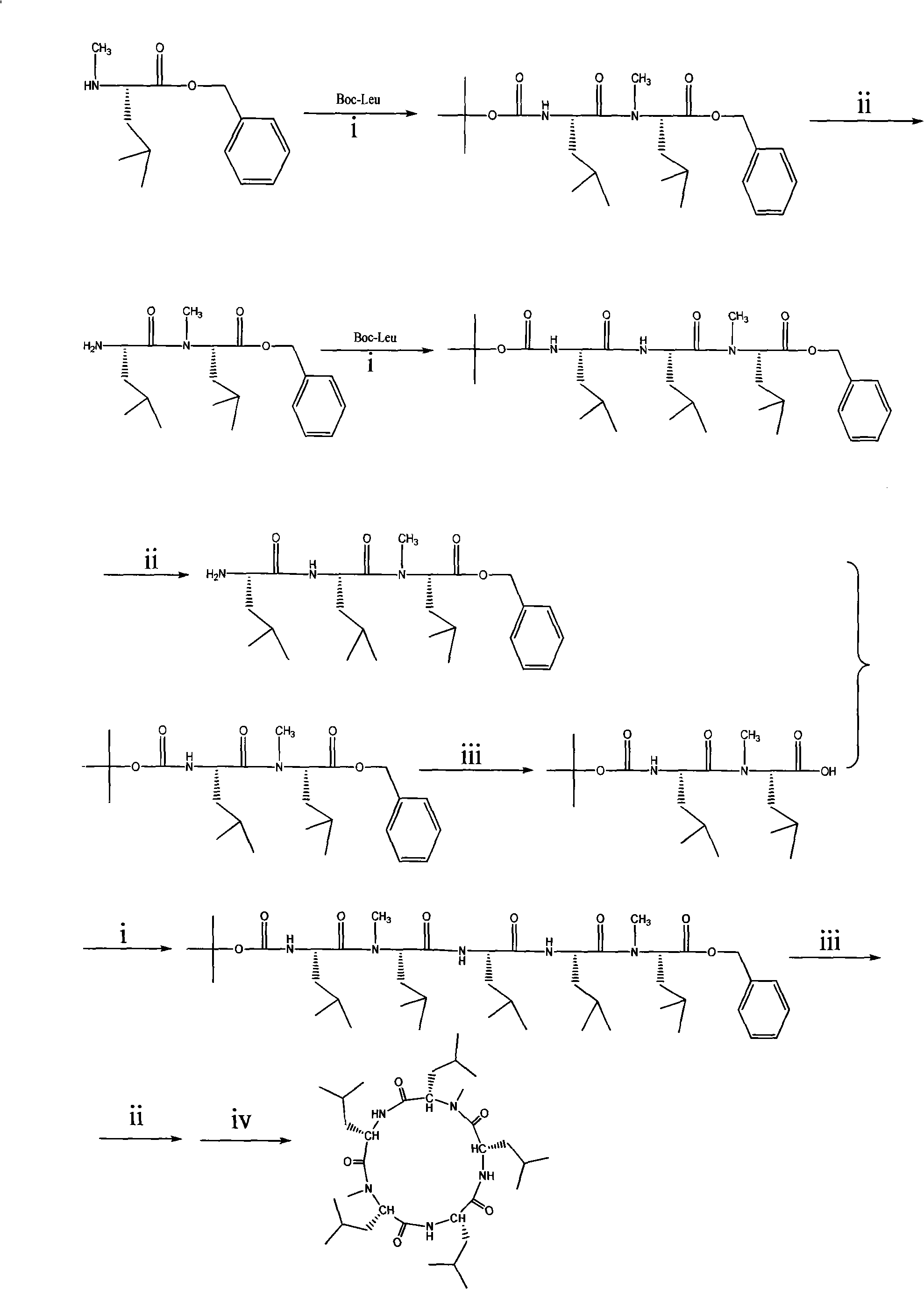Cyclo-pentapeptide and synthesizing method
A synthetic method and technology of cyclic pentapeptide, which is applied in the field of cyclic pentapeptide and its synthesis, can solve the problems of difficult synthesis and decreased yield, and achieve the effects of high product purity, low cost of raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of protective dipeptide (Boc-Leu-N-Me-Leu-OBzl)
[0028] Take N-Me-Leu-OBzl hydrochloride (6mmol, 1.63g) and dissolve it in 15mL of dichloromethane. Under ice bath, use N,N-diisopropylethylamine (DIEA) to adjust PH=7~8, HOBt (6.6 mmol, 0.89 g) and tert-butyloxycarbonyl leucine (6.6 mmol, 1.52 g) were sequentially added, and after stirring for 15 minutes under an ice bath, DCC (6.6 mmol, 1.36 g) was added. The temperature was naturally raised to room temperature, and the reaction was carried out for 18 hours, and then filtered. The filtrate was washed with 10% citric acid, saturated sodium bicarbonate and saturated sodium chloride solution successively, the organic phase was separated, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. After passing through a silica gel column, 2.34 g of a crystalline solid (Boc-Leu-N-Me-Leu-Obzl) was obtained with a yield of 87%.
Embodiment 2
[0029] Example 2 Synthesis of protective tripeptide (Boc-Leu-Leu-N-Me-Leu-OBzl)
[0030] Take (Boc-Leu-N-Me-Leu-Obzl) (4mmol, 1.8g) obtained in Example 1 and add 30mL of dichloromethane and 0.86mL of anisole. Fluoroacetic acid, react under ice bath for 3 hours, followed by TLC. After the reaction is complete, concentrate under reduced pressure, add dichloromethane, concentrate under reduced pressure, repeat three times, and then drain with an oil pump to obtain H--Leu-N-Me-Leu -OBzl. Used directly in the next reaction. Add 10 mL of dichloromethane to the above product, adjust pH to 7-8 with DIEA under ice bath, add HOBt (4.4mmol, 0.59g), tert-butyloxycarbonyl leucine (4.4mol, 1.01g) in sequence, After stirring for 15 minutes under ice bath, DCC (4.4 mmol, 0.91 g) was added. Spontaneously warm to room temperature, after 18 hours of reaction, followed by TLC, after the reaction is complete, filter, the filtrate is washed with 10% citric acid, saturated sodium carbonate and saturated...
Embodiment 3
[0031] Example 3 Preparation of protected pentapeptide (Boc-Leu-N-Me-Leu-Leu-Leu-N-Me-Leu-OBzl)
[0032] (1) Synthesis of H-Leu-Leu-N-Me-Leu-OBzl
[0033] Take the (Boc-Leu-Leu-N-Me-Leu-OBzl) (2mmol, 1.12g) obtained in Example 2, add 30mL of dichloromethane and 0.43mL of anisole, and add 4mL of 20% while stirring under an ice bath. The trifluoroacetic acid was reacted under ice bath for 3 hours, followed by TLC. After the reaction was complete, concentrated under reduced pressure, then added dichloromethane, concentrated under reduced pressure, repeated three times, and then drained with an oil pump to obtain the product (H-Leu-Leu- N-Me-Leu-Obzl) to be used.
[0034] (2) Synthesis of Boc-Leu-N-Me-Leu-OH
[0035] Take the (Boc-Leu-N-Me-Leu-Obzl) (2mmol, 0.90g) obtained in Example 1 and put it into a two-neck round bottom flask, add 20mL of ethyl acetate / methanol (volume ratio=1:1), After being protected by nitrogen gas, 0.3 g of 10% palladium / carbon was added, hydrogen gas was int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com