Method for synthesizing L-theanine with enzyme
An enzymatic synthesis, theanine technology, applied in the field of L-theanine synthesis, can solve the problems of harsh conditions, long fermentation time, many side reactions, etc., and achieve high conversion rate, high conversion rate and simple reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 15g of sucrose, 30g of corn steep liquor, 15g of peptone, K 2 HPO 4 15g, MgSO 4 0.5g, pH 7.5, add tap water to make 1L liquid culture medium, prepare 3.5L according to this recipe, put it into a 5L fermenter and put it into a 0.1MPa high-pressure steam sterilization for 25 minutes for later use. At the same time, divide 50mL of medium into 500mL, add 8 layers of gauze, wrap it in kraft paper, and sterilize it together with the fermenter for later use.
[0033] After taking out the bacterial species preserved in the refrigerator—Bacillus subtillis NX-2 (preservation number is CGMCCNo.0833), connect it to fresh slant medium (slant medium (g / L): peptone 10, beef extract 3. On NaCl 5, agar 20), activate the culture for 24 hours, insert the seed medium (seed medium (g / L): glucose 15, corn steep liquor 10, peptone 10, K 2 HPO 4 2. MgSO 4 0.25) Shaking culture at 33°C and 220r / min for 20 hours. At the end of the cultivation, it was connected to a fermenter, and ferm...
Embodiment 2
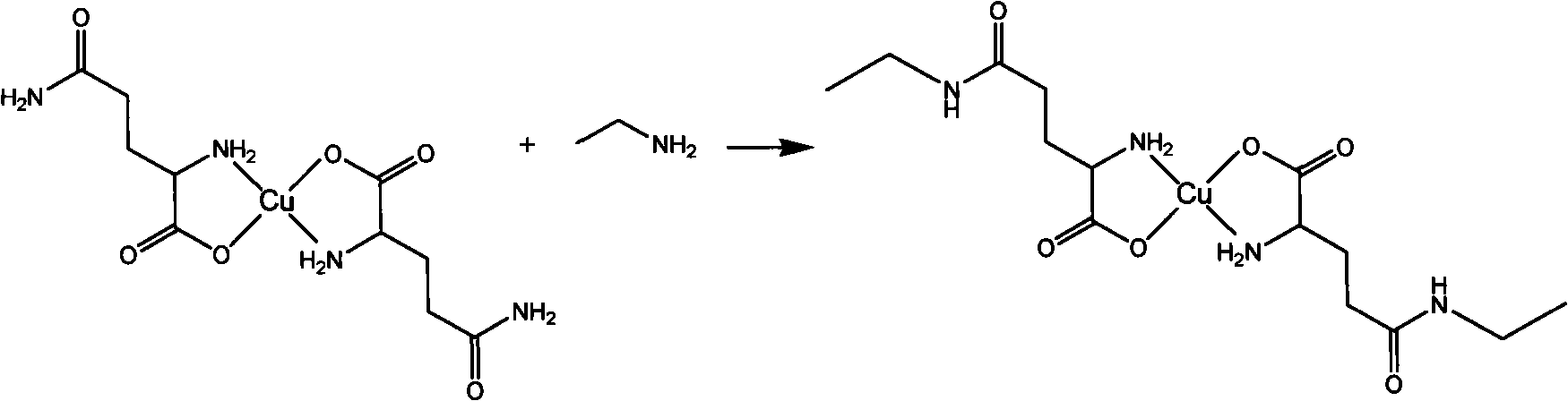
[0036] Weigh 7.3g (0.1mol) of L-glutamine, dissolve 4.2g of sodium bicarbonate in 50mL of water, add 50mL of copper sulfate pentahydrate through a constant flow pump, and control the reaction temperature at about 60°C. After feeding, continue to react at room temperature for about 2 hours, then filter, wash, and dry to obtain L-glutamine-copper (II) complex.
Embodiment 3
[0038]Get 10.6mg (0.03mmol) of the L-glutamine-copper (II) complex obtained in Example 2 in 2.5mL Tris-HCl buffer solution (pH 9, 0.1mol / L), add 2.5mL 400mmol / L Ethylamine, and then add 0.8 mL of γ-glutamyl transpeptidase obtained in Example 1, mix well and place in a water bath for 2 hours of reaction at a reaction temperature of 37°C. After the reaction finished, the reaction solution was added with 1mol / L hydrochloric acid (volume is 10% of the reaction solution) and mixed evenly, and was analyzed and detected with a high performance liquid chromatography system (chromatographic separation condition: the chromatographic column was: DIONEX1Acclaim1205μC18; the detection wavelength was 200nm; the mobile phase was 95 % water (containing 1‰ trifluoroacetic acid), 5% acetonitrile; flow rate: 1mL / min), the conversion rate of glutamine was 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com